Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis
- PMID: 12050120
- PMCID: PMC186321
- DOI: 10.1101/gad.981202
Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis
Abstract
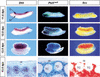
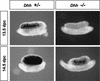
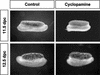
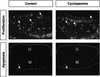
Establishment of the steroid-producing Leydig cell lineage is an event downstream of Sry that is critical for masculinization of mammalian embryos. Neither the origin of fetal Leydig cell precursors nor the signaling pathway that specifies the Leydig cell lineage is known. Based on the sex-specific expression patterns of Desert Hedgehog (Dhh) and its receptor Patched 1 (Ptch1) in XY gonads, we investigated the potential role of DHH/PTCH1 signaling in the origin and specification of fetal Leydig cells. Analysis of Dhh(-/-) XY gonads revealed that differentiation of fetal Leydig cells was severely defective. Defects in Leydig cell differentiation in Dhh(-/-) XY gonads did not result from failure of cell migration from the mesonephros, thought to be a possible source of Leydig cell precursors. Nor did DHH/PTCH1 signaling appear to be involved in the proliferation or survival of fetal Leydig precursors in the interstitium of the XY gonad. Instead, our results suggest that DHH/PTCH1 signaling triggers Leydig cell differentiation by up-regulating Steroidogenic Factor 1 and P450 Side Chain Cleavage enzyme expression in Ptch1-expressing precursor cells located outside testis cords.
Figures


References
-
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. - PubMed
-
- Ariyaratne SHB, Mendis-Handagama CS, Hales BD, Mason IJ. Studies on the onset of Leydig precursor cell differentiation in the prepubertal rat testis. Biol Reprod. 2000;63:165–171. - PubMed
-
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. - PubMed
-
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert Hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. - PubMed
-
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
