Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses
- PMID: 12050360
- PMCID: PMC136273
- DOI: 10.1128/jvi.76.13.6480-6486.2002
Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses
Abstract
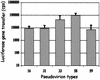
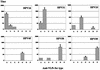
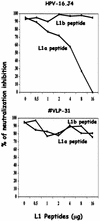
The neutralizing activities of polyclonal antibodies and monoclonal antibodies (MAbs) obtained by immunization of mice with L1 virus-like particles (VLPs) were investigated by using pseudovirion infectivity assays for human papillomavirus type 16 (HPV-16), HPV-31, HPV-33, HPV-45, HPV-58, and HPV-59 to obtain a better definition of cross-neutralization between high-risk HPVs. In this study, we confirmed and extended previous studies indicating that most genital HPV genotypes represent separate serotypes, and the results suggest that the classification of serotypes is similar to that of genotypes. In addition, three cross-neutralizing MAbs were identified (HPV-16.J4, HPV-16.I23, and HPV-33.E12). MAb HPV-16.J4 recognized a conserved linear epitope located within the FG loop of the L1 protein, and HPV-16.I23 recognized another located within the DE loop. The results suggested that reactivity of MAb HPV-16.I23 to L1 protein is lost when leucine 152 of the HPV-16 L1 protein is replaced by phenylalanine. This confirmed the existence of linear epitopes within the L1 protein that induce neutralizing antibodies, and this is the first evidence that such linear epitopes induce cross-neutralization. However, the cross-neutralization induced by L1 VLPs represents less than 1% of the neutralizing activity induced by the dominant conformational epitopes, and it is questionable whether this is sufficient to offer cross-protection in vivo.
Figures
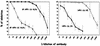

Similar articles
-
Identification of type-specific and cross-reactive neutralizing conformational epitopes on the major capsid protein of human papillomavirus type 31.Arch Virol. 2006 Aug;151(8):1511-23. doi: 10.1007/s00705-006-0734-y. Epub 2006 Mar 3. Arch Virol. 2006. PMID: 16508703 Free PMC article.
-
Mutations on the FG surface loop of human papillomavirus type 16 major capsid protein affect recognition by both type-specific neutralizing antibodies and cross-reactive antibodies.J Med Virol. 2005 Dec;77(4):558-65. doi: 10.1002/jmv.20492. J Med Virol. 2005. PMID: 16254978
-
Hybrid papillomavirus L1 molecules assemble into virus-like particles that reconstitute conformational epitopes and induce neutralizing antibodies to distinct HPV types.Virology. 2001 Dec 20;291(2):324-34. doi: 10.1006/viro.2001.1220. Virology. 2001. PMID: 11878901
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
-
Functional assessment and structural basis of antibody binding to human papillomavirus capsid.Rev Med Virol. 2016 Mar;26(2):115-28. doi: 10.1002/rmv.1867. Epub 2015 Dec 17. Rev Med Virol. 2016. PMID: 26676802 Review.
Cited by
-
Human papillomavirus type 16 pseudovirions with few point mutations in L1 major capsid protein FG loop could escape actual or future vaccination for potential use in gene therapy.Mol Biotechnol. 2014 May;56(5):479-86. doi: 10.1007/s12033-014-9745-1. Mol Biotechnol. 2014. PMID: 24639327
-
Epitope design of L1 protein for vaccine production against Human Papilloma Virus types 16 and 18.Bioinformation. 2017 Mar 31;13(3):86-93. doi: 10.6026/97320630013086. eCollection 2017. Bioinformation. 2017. PMID: 28584449 Free PMC article.
-
Human Papillomavirus (HPV) type 16 and type 18 DNA Loads at Baseline and Persistence of Type-Specific Infection during a 2-year follow-up.J Infect Dis. 2009 Dec 1;200(11):1789-97. doi: 10.1086/647993. J Infect Dis. 2009. PMID: 19848609 Free PMC article.
-
An improved method for estimating antibody titers in microneutralization assay using green fluorescent protein.J Biopharm Stat. 2016;26(3):409-20. doi: 10.1080/10543406.2015.1052475. Epub 2015 May 26. J Biopharm Stat. 2016. PMID: 26010892 Free PMC article.
-
Stability, enrichment, and quantification of total and HPV16-specific IgG present in first-void urine.Sci Rep. 2024 Jun 23;14(1):14441. doi: 10.1038/s41598-024-65257-0. Sci Rep. 2024. PMID: 38910149 Free PMC article.
References
-
- Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmaret, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. - PMC - PubMed
-
- Carter, J. J., L. A. Koutsky, G. C. Wipf, N. D. Christensen, S. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174:927-936. - PubMed
-
- Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus type 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911-1919. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

