Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge
- PMID: 12050371
- PMCID: PMC136282
- DOI: 10.1128/jvi.76.13.6586-6595.2002
Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge
Abstract
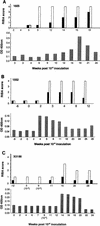
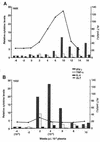
Responses in three chimpanzees were compared following challenge with a clonal hepatitis C virus (HCV) contained in plasma from an animal that had received infectious RNA transcripts. Two of the chimpanzees (Ch1552 and ChX0186) had recovered from a previous infection with HCV, while the third (Ch1605) was a naïve animal. All animals were challenged by reverse titration with decreasing dilutions of plasma and became serum RNA positive following challenge. Ch1605 displayed a typical disease profile for a chimpanzee. We observed increasing levels of serum RNA from week 1 postinoculation (p.i.), reaching a peak of 10(6) copies/ml at week 9 p.i., and alanine aminotransferase (ALT) elevations and seroconversion to HCV antibodies at week 10 p.i. In contrast, both Ch1552 and ChX0186 exhibited much shorter periods of viremia (4 weeks), low serum RNA levels (peak, 10(3) copies/ml), and minimal ALT elevations. A comparison of intrahepatic cytokine levels in Ch1552 and Ch1605 showed greater and earlier gamma interferon (IFN-gamma) and tumor necrosis factor alpha responses in the previously infected animal, responses that were 30-fold greater than baseline responses at week 4 p.i. for IFN-gamma in Ch1552 compared to 12-fold in Ch1605 at week 10 p.i. These data indicate (i) that clonal HCV generated from an infectious RNA transcript will lead to a typical HCV infection in naïve chimpanzees, (ii) that there are memory immune responses in recovered chimpanzees that control HCV infection upon rechallenge, and (iii) that these responses seem to be T-cell mediated, as none of the animals had detectable antibody against the HCV envelope glycoproteins. These observations have encouraging implications for the development of a vaccine for HCV.
Figures
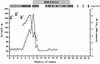




References
-
- Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y. Hu, J. K. Miller, M. A. Gerber, R. E. Sampliner, E. L. Meeks, and M. J. Beach. 1992. The natural history of community-acquired hepatitis C in the United States. N. Engl. J. Med. 327:1899-1905. - PubMed
-
- Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479-1487. - PubMed
-
- Choo, Q. L., G. Kuo, R. Ralston, A. Weiner, D. Chien, G. Van Nest, J. Han, K. Berger, K. Thudium, C. Kuo, J. Kansopon, J. McFarland, A. Tabrizi, K. Ching, B. Moss, L. B. Cummins, M. Houghton, and E. Muchmore. 1994. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 91:1294-1298. - PMC - PubMed
-
- Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-born non-A, non-B viral hepatitis genome. Science 244:359-362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

