Characterization of specific protein-RNA complexes associated with the coupling of polyadenylation and last-intron removal
- PMID: 12052867
- PMCID: PMC133901
- DOI: 10.1128/MCB.22.13.4579-4586.2002
Characterization of specific protein-RNA complexes associated with the coupling of polyadenylation and last-intron removal
Abstract
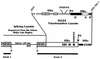
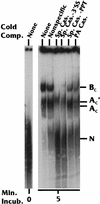
Polyadenylation and splicing are highly coordinated on substrate RNAs capable of coupled polyadenylation and splicing. Individual elements of both splicing and polyadenylation signals are required for the in vitro coupling of the processing reactions. In order to understand more about the coupling mechanism, we examined specific protein-RNA complexes formed on RNA substrates, which undergo coupled splicing and polyadenylation. We hypothesized that formation of a coupling complex would be adversely affected by mutations of either splicing or polyadenylation elements known to be required for coupling. We defined three specific complexes (A(C)', A(C), and B(C)) that form rapidly on a coupled splicing and polyadenylation substrate, well before the appearance of spliced and/or polyadenylated products. The A(C)' complex is formed by 30 s after mixing, the A(C) complex is formed between 1 and 2 min after mixing, and the B(C) complex is formed by 2 to 3 min after mixing. A(C)' is a precursor of A(C), and the A(C)' and/or A(C) complex is a precursor of B(C). Of the three complexes, B(C) appears to be a true coupling complex in that its formation was consistently diminished by mutations or experimental conditions known to disrupt coupling. The characteristics of the A(C)' complex suggest that it is analogous to the spliceosomal A complex, which forms on splicing-only substrates. Formation of the A(C)' complex is dependent on the polypyrimidine tract. The transition from A(C)' to A(C) appears to require an intact 3'-splice site. Formation of the B(C) complex requires both splicing elements and the polyadenylation signal. A unique polyadenylation-specific complex formed rapidly on substrates containing only the polyadenylation signal. This complex, like the A(C)' complex, formed very transiently on the coupled splicing and polyadenylation substrate; we suggest that these two complexes coordinate, resulting in the B(C) complex. We also suggest a model in which the coupling mechanism may act as a dominant checkpoint in which aberrant definition of one exon overrides the normal processing at surrounding wild-type sites.
Figures






References
-
- Berget, S. M. 1995. Exon recognition in vertebrate splicing. J. Biol. Chem. 270:2411-2414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
