Cell attachment to the extracellular matrix induces proteasomal degradation of p21(CIP1) via Cdc42/Rac1 signaling
- PMID: 12052868
- PMCID: PMC133897
- DOI: 10.1128/MCB.22.13.4587-4597.2002
Cell attachment to the extracellular matrix induces proteasomal degradation of p21(CIP1) via Cdc42/Rac1 signaling
Abstract
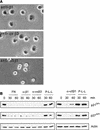
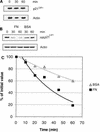
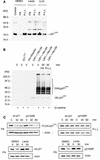
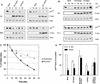
The cyclin-dependent kinase 2 (Cdk2) inhibitors p21(CIP1) and p27(KIP1) are negatively regulated by anchorage during cell proliferation, but it is unclear how integrin signaling may affect these Cdk2 inhibitors. Here, we demonstrate that integrin ligation led to rapid reduction of p21(CIP1) and p27(KIP1) protein levels in three distinct cell types upon attachment to various extracellular matrix (ECM) proteins, including fibronectin (FN), or to immobilized agonistic anti-integrin monoclonal antibodies. Cell attachment to FN did not rapidly influence p21(CIP1) mRNA levels, while the protein stability of p21(CIP1) was decreased. Importantly, the down-regulation of p21(CIP1) and p27(KIP1) was completely blocked by three distinct proteasome inhibitors, demonstrating that integrin ligation induced proteasomal degradation of these Cdk2 inhibitors. Interestingly, ECM-induced proteasomal proteolysis of a ubiquitination-deficient p21(CIP1) mutant (p21K6R) also occurred, showing that the proteasomal degradation of p21(CIP1) was ubiquitin independent. Concomitant with our finding that the small GTPases Cdc42 and Rac1 were activated by attachment to FN, constitutively active (ca) Cdc42 and ca Rac1 promoted down-regulation of p21(CIP1). However, dominant negative (dn) Cdc42 and dn Rac1 mutants blocked the anchorage-induced degradation of p21(CIP1), suggesting that an integrin-induced Cdc42/Rac1 signaling pathway activates proteasomal degradation of p21(CIP1). Our results indicate that integrin-regulated proteasomal proteolysis might contribute to anchorage-dependent cell cycle control.
Figures



References
-
- Blagosklonny, M. V., G. S. Wu, S. Omurand, and W. S. El-Deiry. 1996. Proteasome-dependent regulation of p21WAF1/CIP1 expression. Biochem. Biophys. Res. Commun. 227:564-569. - PubMed
-
- Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56:4620-4624. - PubMed
-
- Brown, J., S. J. Reading, S. Jones, C. J. Fitchett, J. Howl, A. Martin, C. Longland, F. Michelangeli, Y. E. Dubrova, and C. A. Brown. 2000. Critical evaluation of ECV 304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab. Investig. 80:37-45. - PubMed
-
- Chen, C. S., M. Marksich, S. Huang, G. M. Whitesides, and D. E. Ingber. 1997. Geometric control of cell life and death. Science 276:1425-1428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
