Cyclin D and cdk4 are required for normal development beyond the blastula stage in sea urchin embryos
- PMID: 12052892
- PMCID: PMC133905
- DOI: 10.1128/MCB.22.13.4863-4875.2002
Cyclin D and cdk4 are required for normal development beyond the blastula stage in sea urchin embryos
Abstract
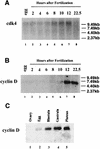
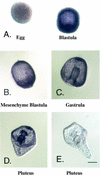
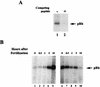
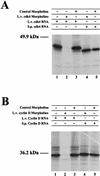
cdk4 mRNA and protein are constitutively expressed in sea urchin eggs and throughout embryonic development. In contrast, cyclin D mRNA is barely detectable in eggs and early embryos, when the cell cycles consist of alternating S and M phases. Cyclin D mRNA increases dramatically in embryos at the early blastula stage and remains at a constant level throughout embryogenesis. An increase in cdk4 kinase activity occurs concomitantly with the increase in cyclin D mRNA. Ectopic expression of cyclin D mRNA in eggs arrests development before the 16-cell stage and causes eventual embryonic death, suggesting that activation of cyclin D/cdk4 in cleavage cell cycles is lethal to the embryo. In contrast, blocking cyclin D or cdk4 expression with morpholino antisense oligonucleotides results in normal development of early gastrula-stage embryos but abnormal, asymmetric larvae. These results suggest that in sea urchins, cyclin D and cdk4 are required for normal development and perhaps the patterning of the developing embryo, but may not be directly involved in regulating entry into the cell cycle.
Figures




References
-
- Audic, Y., B. Boyle, M. Slevin, and R. S. Hartley. 2001. Cyclin E morpholino delays embryogenesis in Xenopus. Genesis 30:107-109. - PubMed
-
- Ceol, C. J., and H. R. Horvitz. 2001. dpl-1 DP and elf-1 E2F act with lin-35 Rb to antagonize ras signaling in C. elegans vulval development. Mol. Cell 3:461-474. - PubMed
-
- De Nooij, J. C., M. A. Letendre, and I. K. Hariharan. 1996. A cyclin-dependent kinase inhibitor, dacapo, is necessary for timely exit from the cell cycle during D. melanogaster embryogenesis. Cell 87:1237-1247. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
