Rad23 promotes the targeting of proteolytic substrates to the proteasome
- PMID: 12052895
- PMCID: PMC133919
- DOI: 10.1128/MCB.22.13.4902-4913.2002
Rad23 promotes the targeting of proteolytic substrates to the proteasome
Abstract
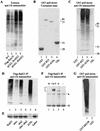
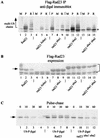
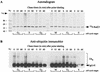
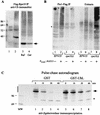
Rad23 contains a ubiquitin-like domain (UbL(R23)) that interacts with catalytically active proteasomes and two ubiquitin (Ub)-associated (UBA) sequences that bind Ub. The UBA domains can bind Ub in vitro, although the significance of this interaction in vivo is poorly understood. Rad23 can interfere with the assembly of multi-Ub chains in vitro, and high-level expression caused stabilization of proteolytic substrates in vivo. We report here that Rad23 interacts with ubiquitinated cellular proteins through the synergistic action of its UBA domains. Rad23 plays an overlapping role with Rpn10, a proteasome-associated multi-Ub chain binding protein. Mutations in the UBA domains prevent efficient interaction with ubiquitinated proteins and result in poor suppression of the growth and proteolytic defects of a rad23 Delta rpn10 Delta mutant. High-level expression of Rad23 revealed, for the first time, an interaction between ubiquitinated proteins and the proteasome. This increase was not observed in rpn10 Delta mutants, suggesting that Rpn10 participates in the recognition of proteolytic substrates that are delivered by Rad23. Overexpression of UbL(R23) caused stabilization of a model substrate, indicating that an unregulated UbL(R23)-proteasome interaction can interfere with the efficient delivery of proteolytic substrates by Rad23. Because the suppression of a rad23 Delta rpn10 Delta mutant phenotype required both UbL(R23) and UBA domains, our findings support the hypothesis that Rad23 encodes a novel regulatory factor that translocates ubiquitinated substrates to the proteasome.
Figures


References
-
- Araki, M., C. Masutani, M. Takemura, A. Uchida, K. Sugawawa, J. Kondoh, Y. Okhuma, and F. Hanaoka. 2001. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 276:18665-18672. - PubMed
-
- Bachmair, A., D. Finley, and A. Varshavsky. 1986. In vivo half-life of a protein is a function of its amino-terminal residue. Science 234:179-186. - PubMed
-
- Beal, R. E., D. Toscano-Cantaffa, P. Young, M. Rechsteiner, and C. M. Pickart. 1998. The hydrophobic effect contributes to polyubiquitin chain recognition. Biochemistry 37:2925-2934. - PubMed
-
- Bertolaet, B. L., D. J. Clarke, M. Wolff, M. H. Watson, M. Henze, G. Divita, and S. I. Reed. 2001. UBA domains mediate protein-protein interactions between two DNA damage-inducible proteins. J. Mol. Biol. 313:955-963. - PubMed
-
- Bertolaet, B. L., D. J. Clarke, M. Wolff, M. H. Watson, M. Henze, G. Divita, and S. I. Reed. 2001. UBA domains of DNA damage-inducible proteins interact with ubiquitin. Nat. Struct. Biol. 8:417-422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
