Intrinsic membrane targeting of the flagellar export ATPase FliI: interaction with acidic phospholipids and FliH
- PMID: 12054792
- PMCID: PMC2528292
- DOI: 10.1016/S0022-2836(02)00172-9
Intrinsic membrane targeting of the flagellar export ATPase FliI: interaction with acidic phospholipids and FliH
Abstract
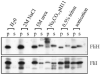
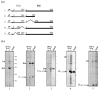
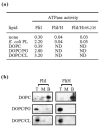
The specialised ATPase FliI is central to export of flagellar axial protein subunits during flagellum assembly. We establish the normal cellular location of FliI and its regulatory accessory protein FliH in motile Salmonella typhimurium, and ascertain the regions involved in FliH(2)/FliI heterotrimerisation. Both FliI and FliH localised to the cytoplasmic membrane in the presence and in the absence of proteins making up the flagellar export machinery and basal body. Membrane association was tight, and FliI and FliH interacted with Escherichia coli phospholipids in vitro, both separately and as the preformed FliH(2)/FliI complex, in the presence or in the absence of ATP. Yeast two-hybrid analysis and pull-down assays revealed that the C-terminal half of FliH (H105-235) directs FliH homodimerisation, and interacts with the N-terminal region of FliI (I1-155), which in turn has an intra-molecular interaction with the remainder of the protein (I156-456) containing the ATPase domain. The FliH105-235 interaction with FliI was sufficient to exert the FliH-mediated down-regulation of ATPase activity. The basal ATPase activity of isolated FliI was stimulated tenfold by bacterial (acidic) phospholipids, such that activity was 100-fold higher than when bound by FliH in the absence of phospholipids. The results indicate similarities between FliI and the well-characterised SecA ATPase that energises general protein secretion. They suggest that FliI and FliH are intrinsically targeted to the inner membrane before contacting the flagellar secretion machinery, with both FliH105-235 and membrane phospholipids interacting with FliI to couple ATP hydrolysis to flagellum assembly.
(c) 2002 Elsevier Science Ltd.
Figures




References
-
- Macnab RM. Flagella and motility. In: Neidhardt FC, editor. Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. 2nd edit. Washington DC: American Society for Microbiology; 1996. pp. 123–145.
-
- Namba K, Vonderviszt F. Molecular architecture of the bacterial flagellum. Quart. Rev. Biophys. 1997;30:1–65. - PubMed
-
- Aizawa SI. Bacterial flagella and type III secretion systems. FEMS Microbiol. Letters. 2001;202:157–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

