Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT(2A) and 5-HT(2C) receptors
- PMID: 12055129
- PMCID: PMC1573376
- DOI: 10.1038/sj.bjp.0704747
Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT(2A) and 5-HT(2C) receptors
Abstract
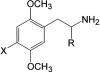
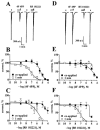
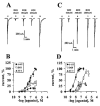
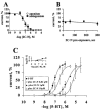
The pharmacological profile of a series of (+/-)-2,5-dimethoxy-4-(X)-phenylisopropylamines (X=I, Br, NO(2), CH(3), or H) and corresponding phenylethylamines, was determined in Xenopus laevis oocytes injected with cRNA coding for rat 5-HT(2A) or 5-HT(2C) receptors. The efficacy and relative potency of these drugs were determined and compared to classical 5-HT(2) receptor agonists and antagonists. The rank order of agonist potency at the 5-HT(2A) receptor was: alpha-methyl-5-HT=5-HT>m-CPP>MK-212; at the 5-HT(2C) receptor the order was: 5-HT>alpha-methyl-5-HT>MK-212>m-CPP. All these compounds were full agonists at the 5-HT(2C) receptor, but alpha-methyl-5-HT and m-CPP showed lower efficacy at the 5-HT(2A) receptor. 4-(4-Fluorobenzoyl)-1-(4-phenylbutyl)piperidine (4F 4PP) was 200 times more potent as a 5-HT(2A) antagonist than at 5-HT(2C) receptors. Conversely, RS 102221 was 100 times more potent as a 5-HT(2C) antagonist, confirming their relative receptor selectivities. The phenylisopropylamines were partial agonists at the 5-HT(2A) receptor, with I(max) relative to 5-HT in the 22+/-7 to 58+/-15% range; the corresponding phenylethylamines had lower or undetectable efficacies. All these drugs had higher efficacies at 5-HT(2C) receptors; DOI was a full 5-HT(2C) agonist. 2C-I and the other phenylethylamines examined showed relative efficacies at the 5-HT(2C) receptor ranging from 44+/-10% to 76+/-16%. 2C-N was a 5-HT(2) receptor antagonist; the mechanism was competitive at the 5-HT(2A), but non-competitive at the 5-HT(2C) receptor. The antagonism was time-dependent at the 5-HT(2C) receptor but independent of pre-incubation time at the 5-HT(2A) receptor subtype. The alpha-methyl group determines the efficacy of these phenylalkylamines at the 5-HT(2A) and 5-HT(2C) receptors.
Figures
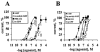


References
-
- ACUÑA C., SCORZA C., REYES-PARADA M., CASSELS B.K., HUIDOBRO-TORO J.P. Aleph-2, a suspected anxiolytic and putative hallucinogenic phenylisopropylamine derivative, is a 5-HT2A and 5-HT2C receptor agonist. Life Sci. 2000;67:3241–3247. - PubMed
-
- ALLES G.A. The comparative physiological actions of dl-β-phenylisopropylamine. II. Bronchial effect. J. Pharmacol. Exp. ther. 1933;48:161–174.
-
- ALLES G.A., PRINZMETAL M. The comparative physiological actions of dl-β-phenylisopropylamine. I. Pressor effect and toxicity. J. Pharmacol. Exper. Therap. 1933;47:339–354.
-
- ARVANOV V.L., LIANG X., RUSSO A., WANG R.Y. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur. J. Neurosci. 1999;11:3064–3072. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

