Enhancement of delayed-rectifier potassium conductance by low concentrations of local anaesthetics in spinal sensory neurones
- PMID: 12055132
- PMCID: PMC1573381
- DOI: 10.1038/sj.bjp.0704754
Enhancement of delayed-rectifier potassium conductance by low concentrations of local anaesthetics in spinal sensory neurones
Abstract
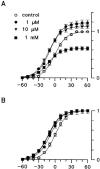
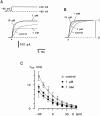
Combining the patch-clamp recordings in slice preparation with the 'entire soma isolation' method we studied action of several local anaesthetics on delayed-rectifier K(+) currents in spinal dorsal horn neurones. Bupivacaine, lidocaine and mepivacaine at low concentrations (1 - 100 microM) enhanced delayed-rectifier K(+) current in intact neurones within the spinal cord slice, while exhibiting a partial blocking effect at higher concentrations (>100 microM). In isolated somata 0.1 - 10 microM bupivacaine enhanced delayed-rectifier K(+) current by shifting its steady-state activation characteristic and the voltage-dependence of the activation time constant to more negative potentials by 10 - 20 mV. Detailed analysis has revealed that bupivacaine also increased the maximum delayed-rectifier K(+) conductance by changing the open probability, rather than the unitary conductance, of the channel. It is concluded that local anaesthetics show a dual effect on delayed-rectifier K(+) currents by potentiating them at low concentrations and partially suppressing at high concentrations. The phenomenon observed demonstrated the complex action of local anaesthetics during spinal and epidural anaesthesia, which is not restricted to a suppression of Na(+) conductance only.
Figures




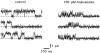
References
-
- ARACAVA Y., IKEDA S.R., DALY J.W., BROOKES N., ALBUQUERQUE E.X. Interactions of bupivacaine with ionic channels of the nicotinic receptor. Analysis of single-channel currents. Mol. Pharmacol. 1984;26:304–313. - PubMed
-
- BARANN M., GOTHERT M., FINK K., BONISCH H. Inhibition by anaesthetics of 14C-guanidinium flux through the voltage-gated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells. Naunyn. Schmiedeberg's Arch. Pharmacol. 1993;347:125–132. - PubMed
-
- BRÄU M.E., KOCH E.D., VOGEL W., HEMPELMANN G. Tonic blocking action of meperidine on Na+ and K+ channels in amphibian peripheral nerves. Anesthesiology. 2000;92:147–155. - PubMed
-
- BRÄU M.E., SANDER F., VOGEL W., HEMPELMANN G. Blocking mechanisms of ketamine and its enantiomers in enzymatically demyelinated peripheral nerve as revealed by single-channel experiments. Anesthesiology. 1997;86:394–404. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

