Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals
- PMID: 12055602
- PMCID: PMC7172544
- DOI: 10.1053/gast.2002.33661
Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals
Abstract
Background & aims: Norwalk Virus (NV) is a member of the Caliciviridae family, which causes acute epidemic gastroenteritis in humans of all ages and its cellular receptors have not yet been characterized. Another calicivirus, Rabbit Hemorrhagic Disease Virus, attaches to H type 2 histo-blood group oligosaccharide present on rabbit epithelial cells. Our aim was to test if, by analogy, recombinant NV-like particles (rNV VLPs) use carbohydrates present on human gastroduodenal epithelial cells as ligands.
Methods: Attachment of rNV VLPs was tested on tissue sections of the gastroduodenal junction and on saliva from individuals of known ABO, Lewis, and secretor phenotypes. It was also tested on human Caco-2 cells and on animal cell lines transfected with glycosyltransferases complementary DNA (cDNA). Competition experiments were performed with synthetic oligosaccharides and anticarbohydrate antibodies. Internalization was monitored by confocal microscopy.
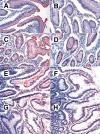
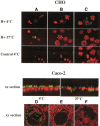
Results: Attachment of rNV VLPs to surface epithelial cells of the gastroduodenal junction as well as to saliva was detected, yet only from secretor donors. It was abolished by alpha1,2fucosidase treatment, and by competition with the H types 1 and 3 trisaccharides or with anti-H type 1 and anti-H types (3/4) antibodies. Transfection of CHO and TS/A cells with an alpha1,2fucosyltransferase cDNA allowed attachment of VLPs. These transfectants as well as differentiated Caco-2 cells expressing H type 1 structures internalized the bound particles.
Conclusions: rNV VLPs use H type 1 and/or H types (3/4) as ligands on gastroduodenal epithelial cells of secretor individuals.
Figures



References
-
- Thiel HJ, König M. Caliciviruses: an overview. Vet Microbiol. 1999;69:55–62. - PubMed
-
- Green K, Ando T, Balayan MS, Berke T, Clarke IN, Estes MK, Matson DO, Nakata S, Neill JD, Studdert MJ, Thiel HJ. Taxonomy of the caliciviruses. J Infect Dis. 2000;181:S322–S330. - PubMed
-
- Shaw RD. Viral infections of the gastrointestinal tract. Curr Opin Gastroenterol. 2000;16:12–17. - PubMed
-
- Glass RI, Noel J, Ando T, Fankhauser R, Belliot G, Mounts A, Parashar UD, Bresee JS, Monroe SS. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J Infect Dis. 2000;181:S254–S261. - PubMed
-
- Koopmans M, Vinjé J, de Witt M, Leenen I, van der Poel W, van Duynhoven Y. Molecular epidemiology of human enteric caliciviruses in the Netherlands. J Infect Dis. 2000;181:S262–S269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

