Gene expression in Wilms' tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition
- PMID: 12057921
- PMCID: PMC1850829
- DOI: 10.1016/S0002-9440(10)61166-2
Gene expression in Wilms' tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition
Abstract
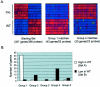
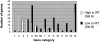
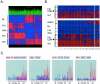
Wilms' tumor (WT) has been considered a prototype for arrested cellular differentiation in cancer, but previous studies have relied on selected markers. We have now performed an unbiased survey of gene expression in WTs using oligonucleotide microarrays. Statistical criteria identified 357 genes as differentially expressed between WTs and fetal kidneys. This set contained 124 matches to genes on a microarray used by Stuart and colleagues (Stuart RO, Bush KT, Nigam SK: Changes in global gene expression patterns during development and maturation of the rat kidney. Proc Natl Acad Sci USA 2001, 98:5649-5654) to establish genes with stage-specific expression in the developing rat kidney. Mapping between the two data sets showed that WTs systematically overexpressed genes corresponding to the earliest stage of metanephric development, and underexpressed genes corresponding to later stages. Automated clustering identified a smaller group of 27 genes that were highly expressed in WTs compared to fetal kidney and heterologous tumor and normal tissues. This signature set was enriched in genes encoding transcription factors. Four of these, PAX2, EYA1, HBF2, and HOXA11, are essential for cell survival and proliferation in early metanephric development, whereas others, including SIX1, MOX1, and SALL2, are predicted to act at this stage. SIX1 and SALL2 proteins were expressed in the condensing mesenchyme in normal human fetal kidneys, but were absent (SIX1) or reduced (SALL2) in cells at other developmental stages. These data imply that the blastema in WTs has progressed to the committed stage in the mesenchymal-epithelial transition, where it is partially arrested in differentiation. The WT-signature set also contained the Wnt receptor FZD7, the tumor antigen PRAME, the imprinted gene NNAT and the metastasis-associated transcription factor E1AF.
Figures




References
-
- Miyagawa K, Kent J, Schedl A, van Heyningen V, Hastie ND: Wilms’ tumour—a case of disrupted development. J Cell Sci 1994, 18:S1-S5 - PubMed
-
- Davies JA, Perera AD, Walker CL: Mechanisms of epithelial development and neoplasia in the metanephric kidney. Int J Dev Biol 1999, 43:473-478 - PubMed
-
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P: Pax-2 controls multiple steps of urogenital development. Development 1995, 121:4057-4065 - PubMed
-
- Sanyanusin P, McNoe LA, Sullivan MJ, Weaver RG, Eccles MR: Mutation of PAX2 in two siblings with renal-coloboma syndrome. Hum Mol Genet 1995, 4:2183-2184 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

