Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype
- PMID: 12057923
- PMCID: PMC1850810
- DOI: 10.1016/S0002-9440(10)61168-6
Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype
Abstract
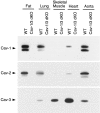
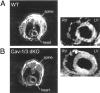
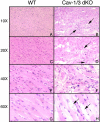
The caveolin gene family consists of caveolins 1, 2, and 3. Caveolins 1 and 2 are co-expressed in many cell types, such as endothelial cells, fibroblasts, smooth muscle cells and adipocytes, where they form a heteroligomeric complex. In contrast, the expression of caveolin-3 is muscle-specific. Thus, the expression of caveolin-1 is required for caveolae formation in non-muscle cells, while the expression of caveolin-3 drives caveolae formation in striated muscle cell types (cardiac and skeletal). To create a truly caveolae-deficient mouse, we interbred Cav-1 null mice and Cav-3 null mice to generate Cav-1/Cav-3 double-knockout (Cav-1/3 dKO) mice. Here, we report that Cav-1/3 dKO mice are viable and fertile, despite the fact that they lack morphologically identifiable caveolae in endothelia, adipocytes, smooth muscle cells, skeletal muscle fibers, and cardiac myocytes. We also show that these mice are deficient in all three caveolin gene products, as caveolin-2 is unstable in the absence of caveolin-1. Interestingly, Cav-1/3 dKO mice develop a severe cardiomyopathy. At 2 months of age, analysis of Cav-1/3 dKO hearts via gated magnetic resonance imaging reveals a dramatic increase in left ventricular wall thickness, as compared with Cav-1-KO, Cav-3 KO, and wild-type mice. Further functional analysis of Cav-1/3 dKO hearts via transthoracic echocardiography demonstrates hypertrophy and dilation of the left ventricle, with a significant decrease in fractional shortening. As predicted, Northern analysis of RNA derived from the left ventricle of Cav-1/3 dKO mice shows a dramatic up-regulation of the atrial natriuretic factor message, a well-established biochemical marker of cardiac hypertrophy. Finally, histological analysis of Cav-1/3 dKO hearts reveals hypertrophy, disorganization, and degeneration of the cardiac myocytes, as well as chronic interstitial fibrosis and inflammation. Thus, dual ablation of both Cav-1 and Cav-3 genes in mice leads to a pleiotropic defect in caveolae formation and severe cardiomyopathy.
Figures



References
-
- Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP: Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins J Biol Chem 1996, 271:15160-15165 - PubMed
-
- Galbiati F, Razani B, Lisanti MP: Role of caveolae and caveolin-3 in muscular dystrophy. Trends in Molecular Medicine 2001, 7:435-441 - PubMed
-
- Galbiati F, Razani B, Lisanti MP: Emerging themes in lipid rafts and caveolae. Cell 2001, 106:403-411 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

