Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis
- PMID: 12058058
- PMCID: PMC117613
- DOI: 10.1091/mbc.02-02-0025
Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis
Abstract
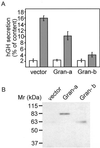
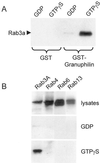
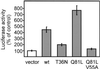
Granuphilin/Slp-4 is a member of the synaptotagmin-like protein family expressed in pancreatic beta-cells and in the pituitary gland. We show by confocal microscopy that both granuphilin-a and -b colocalize with insulin-containing secretory granules positioned at the periphery of pancreatic beta-cells. Overexpression of granuphilins in insulin-secreting cell lines caused a profound inhibition of stimulus-induced exocytosis. Granuphilins were found to bind to two components of the secretory machinery of pancreatic beta-cells, the small GTP-binding protein Rab3 and the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-binding protein Munc-18. The interaction with Rab3 occurred only with the GTP-bound form of the protein and was prevented by a point mutation in the effector domain of the GTPase. Structure-function studies using granuphilin-b mutants revealed that complete loss of Rab3 binding is associated with a reduction in the capacity to inhibit exocytosis. However, the granuphilin/Rab3 complex alone is not sufficient to mediate the decrease of exocytosis, suggesting the existence of additional binding partners. Taken together, our observations indicate that granuphilins play an important role in pancreatic beta-cell exocytosis. In view of the postulated role of Munc-18 in secretory vesicle docking, our data suggest that granuphilins may also be involved in this process.
Figures





References
-
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. - PubMed
-
- Brondyk WH, McKiernan CJ, Burstein ES, Macara IG. Mutants of Rab3A analogous to oncogenic Ras mutants. J Biol Chem. 1993;268:9410–9415. - PubMed
-
- Chung SH, Takai Y, Holz RW. Evidence that the Rab3a-binding protein, rabphilin3a, enhances regulated secretion. J Biol Chem. 1995;270:16714–16717. - PubMed
-
- Coppola T, Hirling H, Perret-Menoud V, Gattesco S, Catsicas S, Joberty G, Macara IG, Regazzi R. Rabphilin dissociated from Rab3 promotes endocytosis through interaction with rabaptin-5. J Cell Sci. 2001;114:1757–1764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

