The G1/S cyclin Cig2p during meiosis in fission yeast
- PMID: 12058071
- PMCID: PMC117626
- DOI: 10.1091/mbc.01-10-0507
The G1/S cyclin Cig2p during meiosis in fission yeast
Abstract
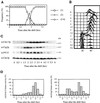
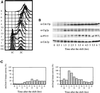
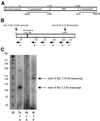
Cyclin-dependent kinases (CDKs) are important for both mitotic and meiotic cell cycles. In fission yeast, the major CDK, Cdc2p is involved in premeiotic DNA replication and in meiosis II. One of its partners, the mitotic cyclin Cdc13p is known to be required for meiosis, whereas there are no studies on the G1/S cyclin Cig2p. In this article, we have studied the regulation of the Cdc2p/Cdc13p and Cdc2p/Cig2p complexes during synchronous meiosis. We observed that Cdc2p/Cig2p kinase is activated in an unexpected biphasic manner, first at onset of premeiotic S phase and again during meiotic nuclear divisions. The role of Cig2p during meiosis was investigated using cig2-deleted strains that exhibit delays in onset of both S phase and meiotic divisions as well as an inefficient completion of MII. Furthermore, analysis of cig2 transcripts revealed a meiosis-specific regulation of cig2 expression during MI/MII dependent upon the Mei4p transcription factor leading to a different transcription start site at this stage of meiosis.
Figures






Similar articles
-
Cyclin B proteolysis and the cyclin-dependent kinase inhibitor rum1p are required for pheromone-induced G1 arrest in fission yeast.Mol Biol Cell. 1998 Jun;9(6):1309-21. doi: 10.1091/mbc.9.6.1309. Mol Biol Cell. 1998. PMID: 9614176 Free PMC article.
-
Felix Hoppe-Seyler Lecture 1999. Cyclin dependent kinases and regulation of the fission yeast cell cycle.Biol Chem. 1999 Jul-Aug;380(7-8):729-33. doi: 10.1515/BC.1999.093. Biol Chem. 1999. PMID: 10494821
-
Feedback regulation of the MBF transcription factor by cyclin Cig2.Nat Cell Biol. 2001 Dec;3(12):1043-50. doi: 10.1038/ncb1201-1043. Nat Cell Biol. 2001. PMID: 11781565
-
Regulation of G1 progression in fission yeast by the rum1+ gene product.Prog Cell Cycle Res. 1996;2:29-35. doi: 10.1007/978-1-4615-5873-6_3. Prog Cell Cycle Res. 1996. PMID: 9552380 Review.
-
CDK Regulation of Meiosis: Lessons from S. cerevisiae and S. pombe.Genes (Basel). 2020 Jun 29;11(7):723. doi: 10.3390/genes11070723. Genes (Basel). 2020. PMID: 32610611 Free PMC article. Review.
Cited by
-
Genomewide identification of pheromone-targeted transcription in fission yeast.BMC Genomics. 2006 Nov 30;7:303. doi: 10.1186/1471-2164-7-303. BMC Genomics. 2006. PMID: 17137508 Free PMC article.
-
The selective elimination of messenger RNA underlies the mitosis-meiosis switch in fission yeast.Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(8):788-97. doi: 10.2183/pjab.86.788. Proc Jpn Acad Ser B Phys Biol Sci. 2010. PMID: 20948174 Free PMC article. Review.
-
Recent advances in understanding of meiosis initiation and the apomictic pathway in plants.Front Plant Sci. 2014 Sep 23;5:497. doi: 10.3389/fpls.2014.00497. eCollection 2014. Front Plant Sci. 2014. PMID: 25295051 Free PMC article. Review.
-
Anaphase-promoting complex/cyclosome-mediated proteolysis of Ams2 in the G1 phase ensures the coupling of histone gene expression to DNA replication in fission yeast.J Biol Chem. 2013 Jan 11;288(2):928-37. doi: 10.1074/jbc.M112.410241. Epub 2012 Nov 29. J Biol Chem. 2013. PMID: 23195958 Free PMC article.
-
Phospho-Regulation of Meiotic Prophase.Front Cell Dev Biol. 2021 Apr 13;9:667073. doi: 10.3389/fcell.2021.667073. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33928091 Free PMC article. Review.
References
-
- Ayté J, Schweitzer C, Zarzov P, Nurse P, DeCaprio JA. Feedback regulation of the MBF transcription factor by cyclin Cig2. Nat Cell Biol. 2001;3:1043–1050. - PubMed
-
- Bähler J, Schuchert P, Grimm C, Kohli J. Synchronized meiosis and recombination in fission yeast: observations with pat1–114 diploid cells. Curr Genet. 1991;19:445–451. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

