Alteration in plasma glucose levels in Japanese encephalitis patients
- PMID: 12059908
- PMCID: PMC2517666
- DOI: 10.1046/j.1365-2613.2002.00213.x
Alteration in plasma glucose levels in Japanese encephalitis patients
Abstract
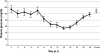
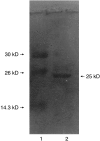
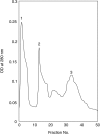
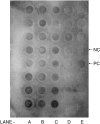
A unique factor, human T cell hypoglycaemic factor (hTCHF), has been shown to produce hypoglycaemia during the convalescent stage in the plasma of patients with Japanese encephalitis virus (JEV) infection. The present study was undertaken to investigate the ability of T cells from fresh peripheral blood mononuclear cells (PBMC) of such patients to produce hTCHF. The PBMC, as well as the individual subpopulations, were cultured for 24 h and the culture supernatants (CS) were assayed for hypoglycaemic activity. The activity was observed in the CD8+ T cells. The hypoglycaemia in JE-confirmed patients coincided with the gradual rise in circulating glucagon level, with no significant alterations in insulin, growth hormone and cortisol levels. The hTCHF was purified by ion exchange chromatography and the purified protein was observed as a approximately 25 kDa band on SDS-PAGE. Secretory hTCHF in the sera of patients and T cell CS was present in 88% of convalescent serum samples. We conclude that during the convalescent stage of JEV infection, a unique factor, hTCHF, is secreted by activated CD8+ T cells from patients and that this is responsible for the development of hypoglycaemia.
Figures


Similar articles
-
Induction of hypoglycaemia in Japanese encephalitis virus infection: the role of T lymphocytes.Clin Exp Immunol. 1997 Feb;107(2):282-7. doi: 10.1111/j.1365-2249.1997.281-ce1173.x. Clin Exp Immunol. 1997. PMID: 9030864 Free PMC article.
-
PD1+CCR2+CD8+ T Cells Infiltrate the Central Nervous System during Acute Japanese Encephalitis Virus Infection.Virol Sin. 2019 Oct;34(5):538-548. doi: 10.1007/s12250-019-00134-z. Epub 2019 Jun 18. Virol Sin. 2019. PMID: 31215000 Free PMC article.
-
Cell-mediated immune responses in healthy children with a history of subclinical infection with Japanese encephalitis virus: analysis of CD4+ and CD8+ T cell target specificities by intracellular delivery of viral proteins using the human immunodeficiency virus Tat protein transduction domain.J Gen Virol. 2004 Feb;85(Pt 2):471-482. doi: 10.1099/vir.0.19531-0. J Gen Virol. 2004. PMID: 14769905
-
Japanese encephalitis virus-specific proliferative responses of human peripheral blood T lymphocytes.Am J Trop Med Hyg. 1995 Sep;53(3):278-83. doi: 10.4269/ajtmh.1995.53.278. Am J Trop Med Hyg. 1995. PMID: 7573713
-
Proliferative response of human peripheral blood mononuclear cells to Japanese encephalitis virus.Microbiol Immunol. 1995;39(4):269-73. doi: 10.1111/j.1348-0421.1995.tb02200.x. Microbiol Immunol. 1995. PMID: 7651240
Cited by
-
Long-term neurological and healthcare burden of adults with Japanese encephalitis: A nationwide study 2000-2015.PLoS Negl Trop Dis. 2021 Sep 14;15(9):e0009703. doi: 10.1371/journal.pntd.0009703. eCollection 2021 Sep. PLoS Negl Trop Dis. 2021. PMID: 34520457 Free PMC article.
References
-
- Burke DS, Lorsomrudee LV, Leake CJ, et al. Fatal outcome in Japanese encephalitis. AmJTropMedHyg. 1985;34:1203–1210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

