C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity
- PMID: 12060712
- PMCID: PMC123169
- DOI: 10.1073/pnas.132128899
C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity
Abstract
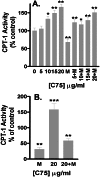
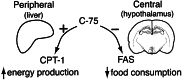
C75, a known inhibitor of fatty acid synthase is postulated to cause significant weight loss through decreased hypothalamic neuropeptide Y (NPY) production. Peripherally, C75, an alpha-methylene-gamma-butyrolactone, reduces adipose tissue and fatty liver, despite high levels of malonyl-CoA. To investigate this paradox, we studied the effect of C75 on fatty acid oxidation and energy production in diet-induced obese (DIO) mice and cellular models. Whole-animal calorimetry showed that C75-treated DIO mice had a 50% greater weight loss, and a 32.9% increased production of energy because of fatty acid oxidation, compared with paired-fed controls. Etomoxir, an inhibitor of carnitine O-palmitoyltransferase-1 (CPT-1), reversed the increased energy expenditure in DIO mice by inhibiting fatty acid oxidation. C75 treatment of rodent adipocytes and hepatocytes and human breast cancer cells increased fatty acid oxidation and ATP levels by increasing CPT-1 activity, even in the presence of elevated concentrations of malonyl-CoA. Studies in human cancer cells showed that C75 competed with malonyl-CoA, as measured by CPT-1 activity assays. Thus, C75 acts both centrally to reduce food intake and peripherally to increase fatty acid oxidation, leading to rapid and profound weight loss, loss of adipose mass, and resolution of fatty liver. The pharmacological stimulation of CPT-1 activity is a novel finding. The dual action of the C75 class of compounds as fatty acid synthase inhibitors and CPT-1 agonists has therapeutic implications in the treatment of obesity and type II diabetes.
Figures



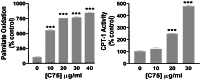
Comment in
-
The search for new ways to treat obesity.Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9096-7. doi: 10.1073/pnas.152327099. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093927 Free PMC article. No abstract available.
Similar articles
-
Chronic C75 treatment of diet-induced obese mice increases fat oxidation and reduces food intake to reduce adipose mass.Am J Physiol Endocrinol Metab. 2004 Jul;287(1):E97-E104. doi: 10.1152/ajpendo.00261.2003. Epub 2004 Jan 21. Am J Physiol Endocrinol Metab. 2004. PMID: 14736702
-
C75 alters central and peripheral gene expression to reduce food intake and increase energy expenditure.Endocrinology. 2005 Jan;146(1):486-93. doi: 10.1210/en.2004-0976. Epub 2004 Oct 21. Endocrinology. 2005. PMID: 15498887
-
Novel effect of C75 on carnitine palmitoyltransferase I activity and palmitate oxidation.Biochemistry. 2006 Apr 11;45(14):4339-50. doi: 10.1021/bi052186q. Biochemistry. 2006. PMID: 16584169
-
Fatty acid metabolism as a target for obesity treatment.Physiol Behav. 2005 May 19;85(1):25-35. doi: 10.1016/j.physbeh.2005.04.014. Physiol Behav. 2005. PMID: 15878185 Review.
-
Fatty acid metabolism, the central nervous system, and feeding.Obesity (Silver Spring). 2006 Aug;14 Suppl 5:201S-207S. doi: 10.1038/oby.2006.309. Obesity (Silver Spring). 2006. PMID: 17021367 Review.
Cited by
-
Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver.Proc Natl Acad Sci U S A. 2004 Apr 27;101(17):6409-14. doi: 10.1073/pnas.0401627101. Epub 2004 Apr 19. Proc Natl Acad Sci U S A. 2004. PMID: 15096593 Free PMC article.
-
Designing the Next Generation of Vaccines: Relevance for Future Pandemics.mBio. 2020 Dec 22;11(6):e02616-20. doi: 10.1128/mBio.02616-20. mBio. 2020. PMID: 33443120 Free PMC article. Review.
-
Stimulation of carnitine palmitoyltransferase 1 improves renal function and attenuates tissue damage after ischemia/reperfusion.J Surg Res. 2012 Sep;177(1):157-64. doi: 10.1016/j.jss.2012.05.053. Epub 2012 Jun 2. J Surg Res. 2012. PMID: 22698429 Free PMC article.
-
The effects of C75, an inhibitor of fatty acid synthase, on sleep and metabolism in mice.PLoS One. 2012;7(2):e30651. doi: 10.1371/journal.pone.0030651. Epub 2012 Feb 13. PLoS One. 2012. PMID: 22348016 Free PMC article.
-
Mice lacking proSAAS display alterations in emotion, consummatory behavior and circadian entrainment.Genes Brain Behav. 2022 Sep;21(7):e12827. doi: 10.1111/gbb.12827. Epub 2022 Jul 25. Genes Brain Behav. 2022. PMID: 35878875 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

