Ultrafast spectroscopy reveals subnanosecond peptide conformational dynamics and validates molecular dynamics simulation
- PMID: 12060746
- PMCID: PMC123009
- DOI: 10.1073/pnas.122238799
Ultrafast spectroscopy reveals subnanosecond peptide conformational dynamics and validates molecular dynamics simulation
Abstract
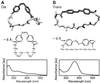
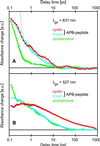
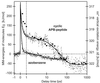
Femtosecond time-resolved spectroscopy on model peptides with built-in light switches combined with computer simulation of light-triggered motions offers an attractive integrated approach toward the understanding of peptide conformational dynamics. It was applied to monitor the light-induced relaxation dynamics occurring on subnanosecond time scales in a peptide that was backbone-cyclized with an azobenzene derivative as optical switch and spectroscopic probe. The femtosecond spectra permit the clear distinguishing and characterization of the subpicosecond photoisomerization of the chromophore, the subsequent dissipation of vibrational energy, and the subnanosecond conformational relaxation of the peptide. The photochemical cis/trans-isomerization of the chromophore and the resulting peptide relaxations have been simulated with molecular dynamics calculations. The calculated reaction kinetics, as monitored by the energy content of the peptide, were found to match the spectroscopic data. Thus we verify that all-atom molecular dynamics simulations can quantitatively describe the subnanosecond conformational dynamics of peptides, strengthening confidence in corresponding predictions for longer time scales.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

