FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication
- PMID: 12060768
- PMCID: PMC123051
- DOI: 10.1073/pnas.122686299
FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication
Abstract
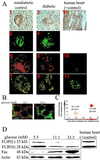
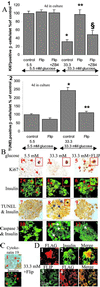
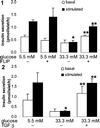
Type 2 diabetes mellitus results from an inadequate adaptation of the functional pancreatic beta cell mass in the face of insulin resistance. Changes in the concentration of glucose play an essential role in the regulation of beta cell turnover. In human islets, elevated glucose concentrations impair beta cell proliferation and induce beta cell apoptosis via up-regulation of the Fas receptor. Recently, it has been shown that the caspase-8 inhibitor FLIP may divert Fas-mediated death signals into those for cell proliferation in lymphatic cells. We observed expression of FLIP in human pancreatic beta cells of nondiabetic individuals, which was decreased in tissue sections of type 2 diabetic patients. In vitro exposure of islets from nondiabetic organ donors to high glucose levels decreased FLIP expression and increased the percentage of apoptotic terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL)-positive beta cells; FLIP was no longer detectable in such TUNEL-positive beta cells. Up-regulation of FLIP, by incubation with transforming growth factor beta or by transfection with an expression vector coding for FLIP, protected beta cells from glucose-induced apoptosis, restored beta cell proliferation, and improved beta cell function. The beneficial effects of FLIP overexpression were blocked by an antagonistic anti-Fas antibody, indicating their dependence on Fas receptor activation. The present data provide evidence for expression of FLIP in the human beta cell and suggest a novel approach to prevent and treat diabetes by switching Fas signaling from apoptosis to proliferation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

