Crystal structure of auxin-binding protein 1 in complex with auxin
- PMID: 12065401
- PMCID: PMC126050
- DOI: 10.1093/emboj/cdf291
Crystal structure of auxin-binding protein 1 in complex with auxin
Abstract
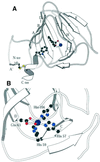
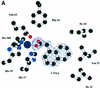
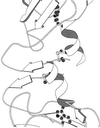
The structure of auxin-binding protein 1 (ABP1) from maize has been determined at 1.9 A resolution, revealing its auxin-binding site. The structure confirms that ABP1 belongs to the ancient and functionally diverse germin/seed storage 7S protein superfamily. The binding pocket of ABP1 is predominantly hydrophobic with a metal ion deep inside the pocket coordinated by three histidines and a glutamate. Auxin binds within this pocket, with its carboxylate binding the zinc and its aromatic ring binding hydrophobic residues including Trp151. There is a single disulfide between Cys2 and Cys155. No conformational rearrangement of ABP1 was observed when auxin bound to the protein in the crystal, but examination of the structure reveals a possible mechanism of signal transduction.
Figures
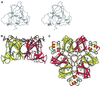



References
-
- Allen F.H. and Kennard,O. (1993) 3D search and research using the Cambridge Structural Database. Chemical Design Automation News, 8, 1 and 31–37.
-
- Anantharaman V., Koonin,E.V. and Aravind,L. (2001) Regulotory potential, phyletic distribution and evolution of ancient, intracellular small-molecule-binding domains. J. Mol. Biol., 307, 1271–1292. - PubMed
-
- Barbier-Brygoo H. Ephritikhine,G., Klambt,D., Maurel,C., Palme,K., Schell,J. and Guern,J. (1991) Perception of the auxin signal at the plasma membrane of tobacco mesophyll protoplasts. Plant J., 1, 83–93. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

