Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway
- PMID: 12065414
- PMCID: PMC126061
- DOI: 10.1093/emboj/cdf306
Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway
Abstract
Drosophila provides a powerful genetic model for studying the in vivo regulation of cell death. In our large-scale gain-of-function screen, we identified Eiger, the first invertebrate tumor necrosis factor (TNF) superfamily ligand that can induce cell death. Eiger is a type II transmembrane protein with a C-terminal TNF homology domain. It is predominantly expressed in the nervous system. Genetic evidence shows that Eiger induces cell death by activating the Drosophila JNK pathway. Although this cell death process is blocked by Drosophila inhibitor-of-apoptosis protein 1 (DIAP1), it does not require caspase activity. We also show genetically that Eiger is a physiological ligand for the Drosophila JNK pathway. Our findings demonstrate that Eiger can initiate cell death through an IAP-sensitive cell death pathway via JNK signaling.
Figures
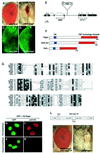
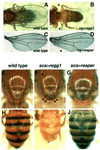
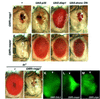
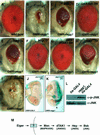


References
-
- Abrams J.M., White,K., Fessler,L.I. and Steller,H. (1993) Programmed cell death during Drosophila embryogenesis. Development, 117, 29–43. - PubMed
-
- Adachi-Yamada T., Fujimura,K.K., Nishida,Y. and Matsumoto,K. (1999) Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature, 400, 166–169. - PubMed
-
- Arnett H.A., Mason,J., Marino,M., Suzuki,K., Matsushima,G.K. and Ting,J.P. (2001) TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nature Neurosci., 4, 1116–1122. - PubMed
-
- Ashkenazi A. and Dixit,V.M. (1998) Death receptors: signaling and modulation. Science, 281, 1305–1308. - PubMed
-
- Chen P., Nordstrom,W., Gish,B. and Abrams,J.M. (1996) grim, a novel cell death gene in Drosophila. Genes Dev., 10, 1773–1782. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

