Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi
- PMID: 12065486
- PMCID: PMC128081
- DOI: 10.1128/IAI.70.7.3468-3478.2002
Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi
Abstract
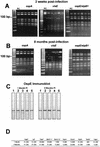
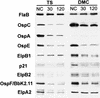
Borrelia burgdorferi differentially expresses many of the OspE/F/Elp paralogs during tick feeding. These findings, combined with the recent report that stable B. burgdorferi infection of mammals occurs only after 53 h of tick attachment, prompted us to further analyze the expression of the OspE/F/Elp paralogs during this critical period of transmission. Indirect immunofluorescence analysis revealed that OspE, p21, ElpB1, ElpB2, and OspF/BbK2.11 are expressed in the salivary glands of ticks allowed to feed on mice for 53 to 58 h. Interestingly, many of the spirochetes in the salivary glands that expressed abundant amounts of these antigens were negative for OspA and OspC. Although prior reports have indicated that OspE/F/Elp orthologs are surface exposed, none of the individual lipoproteins or combinations of the lipoproteins protected mice from challenge infections. To examine why these apparently surface-exposed lipoproteins were not protective, we analyzed their genetic stability during infection and their cellular locations after cultivation in vitro and within dialysis membrane chambers, mimicking a mammalian host-adapted state. Combined restriction fragment length polymorphism and nucleotide sequence analyses revealed that the genes encoding these lipoproteins are stable for at least 8 months postinfection. Interestingly, cellular localization experiments revealed that while all of these proteins can be surface localized, there were significant populations of spirochetes that expressed these lipoproteins only in the periplasm. Furthermore, host-specific signals were found to alter the expression patterns and final cellular location of these lipoproteins. The combined data revealed a remarkable heterogeneity in populations of B. burgdorferi during tick transmission and mammalian infection. The diversity is generated not only by temporal changes in antigen expression but also by modulation of the surface lipoproteins during infection. The ability to regulate the temporal and spatial expression patterns of lipoproteins throughout infection likely contributes to persistent infection of mammals by B. burgdorferi.
Figures


References
-
- Akins, D. R., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homolog. Mol. Microbiol. 18:507-520. - PubMed
-
- Benach, J. L., J. L. Coleman, R. A. Skinner, and E. M. Bosler. 1987. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J. Infect. Dis. 155:1300-1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

