Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8(+)-T-cell populations
- PMID: 12065488
- PMCID: PMC128102
- DOI: 10.1128/IAI.70.7.3493-3499.2002
Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8(+)-T-cell populations
Abstract
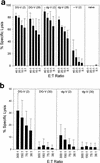
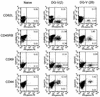
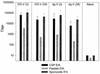
The persistence of immunity to malaria induced in mice by a heterologous DNA priming and poxvirus boosting regimen was characterized. Mice were immunized by priming with DNA vaccine plasmids encoding the Plasmodium yoelii circumsporozoite protein (PyCSP) and murine granulocyte-macrophage colony-stimulating factor and boosting with recombinant vaccinia encoding PyCSP. BALB/c mice immunized with either high-dose (100 microg of p PyCSP plus 30 microg of pGM-CSF) or low-dose (1 microg of p PyCSP plus 1 microg of pGM-CSF DNA) priming were protected against challenge with 50 P. yoelii sporozoites. Protection 2 weeks after immunization was 70 to 100%, persisted at this level for at least 20 weeks, and declined to 30 to 40% by 28 weeks. Eight of eight mice protected at 20 weeks were still protected when rechallenged at 40 weeks. The antigen (Ag)-specific effector CD8(+)-T-cell population present 2 weeks after boosting had ex vivo Ag-specific cytolytic activity, expressed both gamma interferon (IFN-gamma) and tumor necrosis factor alpha, and constituted 12 to 20% of splenic CD8(+) T cells. In contrast, the memory CD8(+)-Ag-specific-cell population at 28 weeks lacked cytolytic activity and constituted only 6% of splenic CD8(+) T cells, but at the single-cell level it produced significantly higher levels of IFN-gamma than the effectors. High levels of Ag- or parasite-specific antibodies present 2 weeks after boosting had declined three- to sevenfold by 28 weeks. Low-dose priming was similarly immunogenic and as protective as high-dose priming against a 50-, but not a 250-, sporozoite challenge. These results demonstrate that a heterologous priming and boosting vaccination can provide lasting protection against malaria in this model system.
Figures

References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Bachmann, M. F., M. Barner, A. Viola, and M. Kopf. 1999. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29:291-299. - PubMed
-
- Charoenvit, Y., S. Mellouk, C. Cole, R. Bechara, M. F. Leef, M. Sedegah, L. F. Yuan, F. A. Robey, R. L. Beaudoin, and S. L. Hoffman. 1991. Monoclonal, but not polyclonal antibodies protect against Plasmodium yoeli sporozoites. J. Immunol. 146:1020-1025. - PubMed
-
- Doolan, D. L., and S. L. Hoffman. 1999. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J. Immunol. 163:884-892. - PubMed
-
- Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453-1462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

