Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells
- PMID: 12065490
- PMCID: PMC128109
- DOI: 10.1128/IAI.70.7.3510-3520.2002
Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells
Abstract
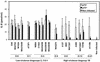
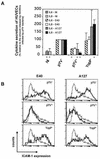
Pathogenic strains of Yersinia spp. inject a set of Yop effector proteins into eukaryotic cells by using a plasmid-encoded type III secretion system. In this study, we analyzed the inflammatory response of human umbilical vein endothelial cells (HUVECs) after infection with different Yersinia enterocolitica strains. We found that both expression of intercellular adhesion molecule 1 and release of the cytokines interleukin-6 (IL-6) and IL-8 by HUVECs are downregulated in a YopP-dependent way, demonstrating that YopP plays a major role in the inflammatory response of these cells. Infection of HUVECs with several low-virulence (biotype 2, 3, and 4) and high-virulence (biotype 1B) Y. enterocolitica strains showed that biotype 1B isolates are more efficient in inhibiting the inflammatory response than low-virulence Y. enterocolitica strains and that this effect depends on the time of contact. We extended the results of Ruckdeschel et al. and found that on the basis of the presence or absence of arginine-143 of YopP (K. Ruckdeschel, K. Richter, O. Mannel, and J. Heesemann, Infect. Immun. 69:7652-7662, 2001) all the Y. enterocolitica strains used fell into two groups, which correlate with the low- and high-virulence phenotypes. In addition, we found that high-virulence strains inject more YopP into the cytosol of eukaryotic target cells than do low-virulence strains.
Figures




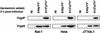
References
-
- Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285-294. - PubMed
-
- Bliska, J. B. 2000. Yop effectors of Yersinia spp. and actin rearrangements. Trends Microbiol. 8:205-208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

