Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation
- PMID: 12065500
- PMCID: PMC128066
- DOI: 10.1128/IAI.70.7.3592-3601.2002
Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation
Abstract
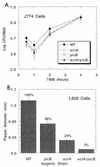
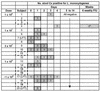
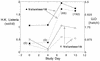
Listeria monocytogenes is an intracellular bacterial pathogen which causes bacteremia and has a tropism for the central nervous system and a propensity to cause maternofetal infection. L. monocytogenes has been shown to be an effective prophylactic and a therapeutic vaccine vector for viral and tumor antigens in animal models. L. monocytogenes mutants lacking the ActA protein, which is essential for intracellular movement, are attenuated but retain immunogenicity in mice. Given the pathogenic potential of L. monocytogenes, we created an attenuated mutant strain bearing double deletions in the actA and plcB virulence genes for an initial clinical safety study of a prototype L. monocytogenes vector in adults. Twenty healthy volunteers received single escalating oral doses (10(6) to 10(9) CFU, 4 volunteers per dose cohort) of this attenuated L. monocytogenes, designated LH1169. Volunteers were monitored in the hospital for 14 days with frequent clinical checks and daily blood and stool cultures, and they were monitored for six additional weeks as outpatients. There were no positive blood cultures and no fevers attributable to the investigational inoculation. Most volunteers shed vaccine bacteria for 4 days or less, without diarrhea. One volunteer had a late positive stool culture during outpatient follow-up. Three volunteers had abnormal liver function test results temporally associated with inoculation; one could be reasonably attributed to another cause. In the highest-dose cohort, humoral, mucosal, and cellular immune responses to the investigational organism were detected in individual volunteers. Attenuated L. monocytogenes can be studied in adult volunteers without serious long-term health sequelae.
Figures



References
-
- Aureli, P., G. C. Fiorucci, D. Caroli, G. Marchiaro, O. Novara, L. Leone, and S. Salmaso. 2000. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes. N. Engl. J. Med. 342:1236-1241. - PubMed
-
- Boury, N. M., and C. J. Czuprynski. 1995. Listeria monocytogenes infection increases neutrophil adhesion and damage to a murine hepatocyte cell line in vitro. Immunol. Lett. 46:111-116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

