The Neisseria lipooligosaccharide-specific alpha-2,3-sialyltransferase is a surface-exposed outer membrane protein
- PMID: 12065517
- PMCID: PMC128106
- DOI: 10.1128/IAI.70.7.3744-3751.2002
The Neisseria lipooligosaccharide-specific alpha-2,3-sialyltransferase is a surface-exposed outer membrane protein
Abstract
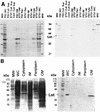
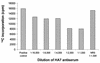
Neisseria gonorrhoeae and Neisseria meningitidis express an approximately 43-kDa alpha-2,3-sialyltransferase (Lst) that sialylates the surface lipooligosaccharide (LOS) by using exogenous (in all N. gonorrhoeae strains and some N. meningitidis serogroups) or endogenous (in other N. meningitidis serogroups) sources of 5'-cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA). Sialylation of LOS can protect N. gonorrhoeae and N. meningitidis from complement-mediated serum killing and from phagocytic killing by neutrophils. The precise subcellular location of Lst has not been determined. We confirm and extend previous studies by demonstrating that Lst is located in the outer membrane and is surface exposed in both N. gonorrhoeae and N. meningitidis. Western immunoblot analysis of subcellular fractions of N. gonorrhoeae strain F62 and N. meningitidis strain MC58 not subset 3 (an acapsulate serogroup B strain) performed with rabbit antiserum raised against recombinant Lst revealed an approximately 43-kDa protein exclusively in outer membrane preparations of both pathogens. Inner membrane, periplasmic, cytoplasmic, and culture supernatant fractions were devoid of Lst, as determined by Western blot analysis. Consistent with this finding, outer membrane fractions of N. gonorrhoeae were significantly enriched for sialyltransferase enzymatic activity. A trace of enzymatic activity was detected in inner membrane fractions, which may have represented Lst in transit to the outer membrane or may have represented inner membrane contamination of outer membrane preparations. Subcellular preparations of an isogenic lst insertion knockout mutant of N. gonorrhoeae F62 (strain ST01) expressed neither a 43-kDa immunoreactive protein nor sialyltransferase activity. Anti-Lst rabbit antiserum bound to whole cells of N. meningitidis MC58 not subset 3 and wild-type N. gonorrhoeae F62 but not to the Lst mutant ST01, indicating the surface exposure of the enzyme. Although the anti-Lst antiserum avidly bound enzymatically active, recombinant Lst, it inhibited Lst (sialyltransferase) activity by only about 50% at the highest concentration of antibody used. On the contrary, anti-Lst antiserum did not inhibit sialylation of whole N. gonorrhoeae cells in the presence of exogenous CMP-NANA, suggesting that the antibody did not bind to or could not access the enzyme active site on the surface of viable Neisseria cells. Taken together, these results indicate that Lst is an outer membrane, surface-exposed glycosyltransferase. To our knowledge, this is the first demonstration of the localization of a bacterial glycosyltransferase to the outer membrane of gram-negative bacteria.
Figures
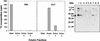

References
-
- Gao, L., L. Linden, N. J. Parsons, J. A. Cole, and H. Smith. 2000. Uptake of metabolites by gonococci grown with lactate in a medium containing glucose: evidence for a surface location of the sialyltransferase. Microb. Pathog. 28:257-266. - PubMed
-
- Gilbert, M., A. M. Cunningham, D. C. Watson, A. Martin, J. C. Richards, and W. W. Wakarchuk. 1997. Characterization of a recombinant Neisseria meningitidis alpha-2,3-sialyltransferase and its acceptor specificity. Eur. J. Biochem. 249:187-194. - PubMed
-
- Gilbert, M., D. C. Watson, A. M. Cunningham, M. P. Jennings, N. M. Young, and W. W. Wakarchuk. 1996. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J. Biol. Chem. 271:28271-28276. - PubMed
-
- Jennings, M. P., D. W. Hood, I. R. Peak, M. Virji, and E. R. Moxon. 1995. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol. Microbiol. 18:729-740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

