Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells
- PMID: 12065523
- PMCID: PMC128046
- DOI: 10.1128/IAI.70.7.3793-3803.2002
Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells
Abstract
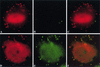
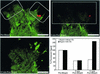
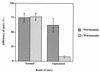
To elucidate the host cell machinery utilized by Chlamydia trachomatis to invade epithelial cells, we examined the role of the actin cytoskeleton in the internalization of chlamydial elementary bodies (EBs). Treatment of HeLa cells with cytochalasin D markedly inhibited the internalization of C. trachomatis serovar L2 and D EBs. Association of EBs with HeLa cells induced localized actin polymerization at the site of attachment, as visualized by either phalloidin staining of fixed cells or the active recruitment of GFP-actin in viable infected cells. The recruitment of actin to the specific site of attachment was accompanied by dramatic changes in the morphology of cell surface microvilli. Ultrastructural studies revealed a transient microvillar hypertrophy that was dependent upon C. trachomatis attachment, mediated by structural components on the EBs, and cytochalasin D sensitive. In addition, a mutant CHO cell line that does not support entry of C. trachomatis serovar L2 did not display such microvillar hypertrophy following exposure to L2 EBs, which is in contrast to infection with serovar D, to which it is susceptible. We propose that C. trachomatis entry is facilitated by an active actin remodeling process that is induced by the attachment of this pathogen, resulting in distinct microvillar reorganization throughout the cell surface and the formation of a pedestal-like structure at the immediate site of attachment and entry.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

