Progressive bovine paratuberculosis is associated with local loss of CD4(+) T cells, increased frequency of gamma delta T cells, and related changes in T-cell function
- PMID: 12065529
- PMCID: PMC128076
- DOI: 10.1128/IAI.70.7.3856-3864.2002
Progressive bovine paratuberculosis is associated with local loss of CD4(+) T cells, increased frequency of gamma delta T cells, and related changes in T-cell function
Abstract
Bovine paratuberculosis is caused by the infection of young calves with Mycobacterium avium subsp. paratuberculosis, resulting in a chronic granulomatous infection of predominantly the ileum. After an incubation period of 2 to 5 years, the disease becomes progressive in some of the chronically infected, but asymptomatic cows. This results in a protein-losing enteropathy that will ultimately be fatal. A loss of cell-mediated immune responses in symptomatic animals has been described, but no information is available concerning immune reactivity in the intestine. We sought to investigate putative disease status-associated lymphocyte subset distributions and antigen-specific functional characteristics of mononuclear cells isolated from blood, gut-associated lymphoid tissue, and the intestinal walls of 22 cows in different stages of disease and in control animals. The results demonstrated a significant decrease in CD4(+) T-cell frequency and a significant increase in TcR1-N12(+) gamma delta T-cell frequency in ileum lamina propria lymphocytes of symptomatic animals compared to the asymptomatic shedders. Immunohistology revealed that there was also an absolute decrease in the number of CD4(+) T cells in sections of the lesional ileum. Our findings also indicated that both peripheral and intestinal cell-mediated responses are decreased in symptomatic animals compared to asymptomatic animals. We conclude that the decrease in cell-mediated responses is likely related to a loss of antigen-specific CD4(+) T cells, which is most prominent in the lesional ileum from symptomatic animals, thus contributing to the progressive nature of bovine paratuberculosis.
Figures
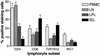
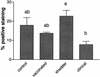

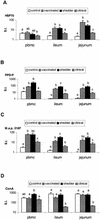
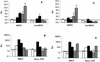

Similar articles
-
Host responses to persistent Mycobacterium avium subspecies paratuberculosis infection in surgically isolated bovine ileal segments.Clin Vaccine Immunol. 2013 Feb;20(2):156-65. doi: 10.1128/CVI.00496-12. Epub 2012 Dec 5. Clin Vaccine Immunol. 2013. PMID: 23221000 Free PMC article.
-
Parturition invokes changes in peripheral blood mononuclear cell populations in Holstein dairy cows naturally infected with Mycobacterium avium subsp. paratuberculosis.Vet Immunol Immunopathol. 2008 Jul 15;124(1-2):50-62. doi: 10.1016/j.vetimm.2008.01.006. Epub 2008 Jan 19. Vet Immunol Immunopathol. 2008. PMID: 18294700
-
ZAP-70, CTLA-4 and proximal T cell receptor signaling in cows infected with Mycobacterium avium subsp. paratuberculosis.Vet Immunol Immunopathol. 2015 Sep 15;167(1-2):15-21. doi: 10.1016/j.vetimm.2015.06.017. Epub 2015 Jul 2. Vet Immunol Immunopathol. 2015. PMID: 26163934
-
Antigen-specific regulatory T cells in bovine paratuberculosis.Vet Immunol Immunopathol. 2008 Oct 15;125(3-4):234-45. doi: 10.1016/j.vetimm.2008.05.019. Epub 2008 Jul 3. Vet Immunol Immunopathol. 2008. PMID: 18602164 Review.
-
Transitions in immune responses to Mycobacterium paratuberculosis.Vet Microbiol. 2000 Dec 20;77(3-4):465-73. doi: 10.1016/s0378-1135(00)00331-x. Vet Microbiol. 2000. PMID: 11118731 Review.
Cited by
-
Differential cytokine gene expression profiles in the three pathological forms of sheep paratuberculosis.BMC Vet Res. 2007 Aug 14;3:18. doi: 10.1186/1746-6148-3-18. BMC Vet Res. 2007. PMID: 17697353 Free PMC article.
-
Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves.Infect Immun. 2004 Dec;72(12):6870-83. doi: 10.1128/IAI.72.12.6870-6883.2004. Infect Immun. 2004. PMID: 15557608 Free PMC article.
-
Predicting the Role of IL-10 in the Regulation of the Adaptive Immune Responses in Mycobacterium avium Subsp. paratuberculosis Infections Using Mathematical Models.PLoS One. 2015 Nov 30;10(11):e0141539. doi: 10.1371/journal.pone.0141539. eCollection 2015. PLoS One. 2015. PMID: 26619346 Free PMC article.
-
Identification of immune parameters to differentiate disease states among sheep infected with Mycobacterium avium subsp. paratuberculosis.Clin Vaccine Immunol. 2010 Jan;17(1):108-17. doi: 10.1128/CVI.00359-09. Epub 2009 Nov 18. Clin Vaccine Immunol. 2010. PMID: 19923568 Free PMC article.
-
Local assessment of the immunohistochemical expression of Foxp3+ regulatory T lymphocytes in the different pathological forms associated with bovine paratuberculosis.BMC Vet Res. 2022 Aug 4;18(1):299. doi: 10.1186/s12917-022-03399-x. BMC Vet Res. 2022. PMID: 35927759 Free PMC article.
References
-
- Abbas, A., K. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. - PubMed
-
- Alzuherri, H. M., C. J. Woodall, and C. J. Clarke. 1996. Increased intestinal TNF-α, IL-1β and IL-6 expression in ovine paratuberculosis. Vet. Immunol. Immunopathol. 49:331-345. - PubMed
-
- Baldwin, C. L., A. J. Teale, J. G. Naessens, B. M. Goddeeris, N. D. MacHugh, and W. I. Morrison. 1986. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J. Immunol. 136:4385-4391. - PubMed
-
- Bendixen, P. H. 1978. Immunological reactions caused by infection with Mycobacterium paratuberculosis: a review. Nord. Vet. Med. 30:163-168. - PubMed
-
- Bloom, B. R., P. Salgame, and B. Diamond. 1992. Revisiting and revising suppressor T cells. Immunol. Today 13:131-136. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

