Differential susceptibility to acute Trypanosoma cruzi infection in BALB/c and C57BL/6 mice is not associated with a distinct parasite load but cytokine abnormalities
- PMID: 12067296
- PMCID: PMC1906265
- DOI: 10.1046/j.1365-2249.2002.01874.x
Differential susceptibility to acute Trypanosoma cruzi infection in BALB/c and C57BL/6 mice is not associated with a distinct parasite load but cytokine abnormalities
Abstract
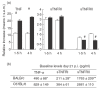
Inoculation of Trypanosoma cruzi, Tulahuén strain, into C57BL/6 and BALB/c mice led to an acute infection characterized by marked parasitaemia, myocardial inflammation and thymocyte depletion. While C57BL/6 mice showed a progressive and lethal disease, BALB/c mice partly recovered. To characterize these murine models more effectively, we studied the parasite burden, serum levels of major infection outcome-related cytokines, the in vitro features of T. cruzi infection in peritoneal macrophages and the immunophenotype of thymic cells. The greater disease severity of T. cruzi-infected C57BL/6 mice was not linked to an increased parasite load, as parasitaemia, myocardial parasite nests and amastigote counts in peritoneal macrophages were not different from those in BALB/c mice. Cortical thymocyte loss was accompanied by the presence of apoptotic bodies and fragmented nuclear DNA, whereas fluorocytometric analysis at 17 days postinfection (p.i.) revealed a more pronounced loss of CD4+ CD8+ cells in C57BL/6 mice. This group displayed higher levels of TNF-alpha on days 14 and 21 p.i., in the presence of lower IL-1beta and IL-10 concentrations by days 14 and 21, and days 7 and 14 p.i., respectively. Day-21 evaluation showed higher concentrations of nitrate and TNF-alpha soluble receptors in C57BL/6 mice with no differences in IFN-gamma levels, with respect to the BALB/c group. Increased morbidity of C57BL/6 T. cruzi-infected mice does not seem to result from an aggravated infection but from an unbalanced relationship between pro- and anti-inflammatory mediators.
Figures
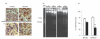
Similar articles
-
Thymocyte depletion during acute Trypanosoma cruzi infection in C57BL/6 mice is partly reverted by lipopolysaccharide pretreatment.FEMS Immunol Med Microbiol. 2004 Jun 1;41(2):123-31. doi: 10.1016/j.femsim.2004.02.003. FEMS Immunol Med Microbiol. 2004. PMID: 15145456
-
[TH1 response in the experimental infection with Trypanosoma cruzi].Medicina (B Aires). 1999;59 Suppl 2:84-90. Medicina (B Aires). 1999. PMID: 10668248 Spanish.
-
CCL3/Macrophage Inflammatory Protein-1α Is Dually Involved in Parasite Persistence and Induction of a TNF- and IFNγ-Enriched Inflammatory Milieu in Trypanosoma cruzi-Induced Chronic Cardiomyopathy.Front Immunol. 2020 Mar 3;11:306. doi: 10.3389/fimmu.2020.00306. eCollection 2020. Front Immunol. 2020. PMID: 32194558 Free PMC article.
-
[Role of cytokines in resistance and pathology in Trypanosoma cruzi infection].Rev Argent Microbiol. 1996 Apr-Jun;28(2):99-109. Rev Argent Microbiol. 1996. PMID: 8768488 Review. Spanish.
-
Immunopathology of cardiomyopathy in the experimental Chagas disease.Mem Inst Oswaldo Cruz. 1999;94 Suppl 1:257-62. doi: 10.1590/s0074-02761999000700043. Mem Inst Oswaldo Cruz. 1999. PMID: 10677729 Review.
Cited by
-
Tumor necrosis factor-α regulates glucocorticoid synthesis in the adrenal glands of Trypanosoma cruzi acutely-infected mice. the role of TNF-R1.PLoS One. 2013 May 22;8(5):e63814. doi: 10.1371/journal.pone.0063814. Print 2013. PLoS One. 2013. PMID: 23717489 Free PMC article.
-
Deficiency in mannose-binding lectin-associated serine protease-2 does not increase susceptibility to Trypanosoma cruzi infection.Am J Trop Med Hyg. 2015 Feb;92(2):320-4. doi: 10.4269/ajtmh.14-0236. Epub 2014 Dec 29. Am J Trop Med Hyg. 2015. PMID: 25548381 Free PMC article.
-
Reciprocal influences between leptin and glucocorticoids during acute Trypanosoma cruzi infection.Med Microbiol Immunol. 2013 Oct;202(5):339-52. doi: 10.1007/s00430-013-0294-1. Epub 2013 May 16. Med Microbiol Immunol. 2013. PMID: 23677171
-
Immune response triggered by Trypanosoma cruzi infection strikes adipose tissue homeostasis altering lipid storage, enzyme profile and adipokine expression.Med Microbiol Immunol. 2019 Oct;208(5):651-666. doi: 10.1007/s00430-018-0572-z. Epub 2018 Nov 9. Med Microbiol Immunol. 2019. PMID: 30413884
-
Role of Aryl Hydrocarbon Receptor (AhR) in the Regulation of Immunity and Immunopathology During Trypanosoma cruzi Infection.Front Immunol. 2019 Mar 29;10:631. doi: 10.3389/fimmu.2019.00631. eCollection 2019. Front Immunol. 2019. PMID: 30984194 Free PMC article.
References
-
- Kirchhoff LV. American trypanosomiasis (Chagas’ disease). A tropical disease now in the United States. N Engl J Med. 1993;329:639–44. - PubMed
-
- Reed SG. In vivo administration of recombinant IFN-γ induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988;140:4342–7. - PubMed
-
- Torrico F, Heremans H, Rivera MT, Marck EV, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–32. - PubMed
-
- Revelli S, Davila H, Ferro ME, et al. Experimental Trypanosoma cruzi infection in the rat. Response to systemic treatment with recombinant rat interferon gamma. Microbiol Immunol. 1995;39:275–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

