Characterization of autoantibodies from patients with Goodpasture's disease using a resonant mirror biosensor
- PMID: 12067312
- PMCID: PMC1906247
- DOI: 10.1046/j.1365-2249.2002.01867.x
Characterization of autoantibodies from patients with Goodpasture's disease using a resonant mirror biosensor
Abstract
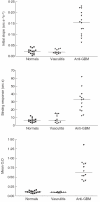
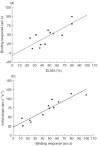
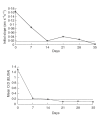
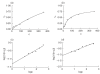
Goodpasture's disease is characterized by the binding of IgG autoantibodies to the glomerular basement membrane, leading to glomerular inflammation. The autoantigen has been identified as the noncollagenous domain of the alpha3 chain of type IV collagen (alpha3(IV)NC1). We have used the IAsys resonant mirror biosensor to analyse the extent and affinity of binding of anti-GBM antibodies from sera of patients to purified alpha3(IV) NC1. alpha3(IV) NC1 monomers were immobilized to a carboxylate cuvette, with the simultaneous use of a control well. The binding of serum from patients with Goodpasture's disease (n = 12), normal controls (n = 14) and disease controls with vasculitis (n = 14) was analysed. Antibody binding was detected in sera from all patients with Goodpasture's disease but not from controls. IAsys measurements of binding correlated with antibody levels assessed by the standardized ELISA used for clinical assays. Both ELISA and biosensor measurements showed declining antibody levels in serial serum samples from treated patients; however, the biosensor detected antibody recrudescence when ELISA remained negative. Autoantibodies from patients' serum had average affinity constants (Kd) of 6.5 x 10-11M to 52.07 x 10-10M, as determined by an inhibition assay, indicating high affinity. Sips analysis showed that the antibody response was relatively homogeneous (values of 0.46-1). Biosensor techniques can therefore be used to detect and characterize anti-GBM antibodies in serum from patients, with high sensitivity and without need for antibody purification. This technique may be useful in diagnosis and monitoring of patients with Goodpasture's disease, and may be applicable to other autoantibody mediated diseases.
Figures

Similar articles
-
High affinity of anti-GBM antibodies from Goodpasture and transplanted Alport patients to alpha3(IV)NC1 collagen.Kidney Int. 2000 Jul;58(1):115-22. doi: 10.1046/j.1523-1755.2000.00146.x. Kidney Int. 2000. PMID: 10886555
-
Antibodies to α5 chain of collagen IV are pathogenic in Goodpasture's disease.J Autoimmun. 2016 Jun;70:1-11. doi: 10.1016/j.jaut.2016.04.001. Epub 2016 Apr 23. J Autoimmun. 2016. PMID: 27117167 Free PMC article.
-
Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis.N Engl J Med. 2010 Jul 22;363(4):343-54. doi: 10.1056/NEJMoa0910500. N Engl J Med. 2010. PMID: 20660402 Free PMC article.
-
Molecular characterization of the target antigens of anti-glomerular basement membrane antibody disease.Springer Semin Immunopathol. 2003 May;24(4):345-61. doi: 10.1007/s00281-002-0103-1. Springer Semin Immunopathol. 2003. PMID: 12778332 Review.
-
Cutting edge issues in Goodpasture's disease.Clin Rev Allergy Immunol. 2011 Oct;41(2):151-62. doi: 10.1007/s12016-010-8222-2. Clin Rev Allergy Immunol. 2011. PMID: 21207195 Review.
Cited by
-
Pulmonary-renal vasculitic disorders: differential diagnosis and management.Curr Rheumatol Rep. 2003 Apr;5(2):107-15. doi: 10.1007/s11926-003-0038-0. Curr Rheumatol Rep. 2003. PMID: 12628041 Review.
-
Basement membranes and autoimmune diseases.Matrix Biol. 2017 Jan;57-58:149-168. doi: 10.1016/j.matbio.2016.07.008. Epub 2016 Aug 2. Matrix Biol. 2017. PMID: 27496347 Free PMC article. Review.
References
-
- Levy JB, Turner AN, Rees AJ, Pusey CD. Long term outcome of anti-GBM antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–42. - PubMed
-
- Saus J, Wieslander J, Langeveld JPM, Quinones S, Hudson BG. Dentification of the Goodpasture antigen as the α3 (IV) chain of collagen IV. J Biol Chem. 1988;263:13374–80. - PubMed
-
- Peters DK, Rees AJ, Lockwood CM, Pusey CD. Treatment and prognosis in antibasement membrane antibody-mediated nephritis. Transplant Proc. 1982;14:513–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

