Convergent and reciprocal modulation of a leak K+ current and I(h) by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones
- PMID: 12068035
- PMCID: PMC2290347
- DOI: 10.1113/jphysiol.2002.018119
Convergent and reciprocal modulation of a leak K+ current and I(h) by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones
Abstract
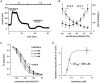
Neurotransmitters and volatile anaesthetics have opposing effects on motoneuronal excitability which appear to reflect contrasting modulation of two types of subthreshold currents. Neurotransmitters increase motoneuronal excitability by inhibiting TWIK-related acid-sensitive K+ channels (TASK) and shifting activation of a hyperpolarization-activated cationic current (I(h)) to more depolarized potentials; on the other hand, anaesthetics decrease excitability by activating a TASK-like current and inducing a hyperpolarizing shift in I(h) activation. Here, we used whole-cell recording from motoneurones in brainstem slices to test if neurotransmitters (serotonin (5-HT) and noradrenaline (NA)) and an anaesthetic (halothane) indeed compete for modulation of the same ion channels - and we determined which prevails. When applied together under current clamp conditions, 5-HT reversed anaesthetic-induced membrane hyperpolarization and increased motoneuronal excitability. Under voltage clamp conditions, 5-HT and NA overcame most, but not all, of the halothane-induced current. When I(h) was blocked with ZD 7288, the neurotransmitters completely inhibited the K+ current activated by halothane; the halothane-sensitive neurotransmitter current reversed at the equilibrium potential for potassium (E(K)) and displayed properties expected of acid-sensitive, open-rectifier TASK channels. To characterize modulation of I(h) in relative isolation, effects of 5-HT and halothane were examined in acidified bath solutions that blocked TASK channels. Under these conditions, 5-HT and halothane each caused their characteristic shift in voltage-dependent gating of I(h). When tested concurrently, however, halothane decreased the neurotransmitter-induced depolarizing shift in I(h) activation. Thus, halothane and neurotransmitters converge on TASK and I(h) channels with opposite effects; transmitter action prevailed over anaesthetic effects on TASK channels, but not over effects on I(h). These data suggest that anaesthetic actions resulting from effects on either TASK or hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in motoneurones, and perhaps at other CNS sites, can be modulated by prevailing neurotransmitter tone.
Figures




References
-
- Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Consciousness and Cognition. 2000;9:370–386. - PubMed
-
- Anzawa N, Kushikata T, Ohkawa H, Yoshida H, Kubota T, Matsuki A. Increased noradrenaline release from rat preoptic area during and after sevoflurane and isoflurane anesthesia. Canadian Journal of Anesthesia. 2001;48:462–465. - PubMed
-
- Bayliss DA, Viana F, Bellingham MC, Berger AJ. Characteristics and postnatal development of a hyperpolarization-activated inward current in rat hypoglossal motoneurons in vitro. Journal of Neurophysiology. 1994;71:119–128. - PubMed
-
- Bazil CW, Minneman KP. Effects of clinically effective concentrations of halothane on adrenergic and cholinergic synapses in rat brain in vitro. Journal of Pharmacology and Experimental Therapeutics. 1989;248:143–148. - PubMed
-
- Berg-Johnsen J, Langmoen IA. Mechanisms concerned in the direct effect of isofluorane on rat hippocampal and human neocortical neurons. Brain Research. 1990;507:28–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

