Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine
- PMID: 12068041
- PMCID: PMC2290375
- DOI: 10.1113/jphysiol.2002.018796
Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine
Abstract
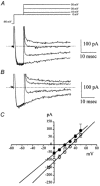
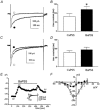
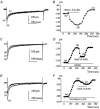
Electrical slow waves in gastrointestinal (GI) muscles are generated by pacemaker cells, known as interstitial cells of Cajal (ICC). The pacemaker conductance is regulated by periodic release of Ca2+ from inositol 1,4,5-trisphosphate (IP(3)) receptor-operated stores, but little is known about how slow waves are actively propagated. We investigated voltage-dependent Ca2+ currents in cultured ICC from the murine colon and small intestine. ICC, identified by kit immunohistochemistry, were spontaneously active under current clamp and generated transient inward (pacemaker) currents under voltage clamp. Depolarization activated inward currents due to entry of Ca2+. Nicardipine (1 microM) blocked only half of the voltage-dependent inward current. After nicardipine, there was a shift in the potential at which peak current was obtained (-15 mV), and negative shifts in the voltage dependence of activation and inactivation of the remaining voltage-dependent inward current. The current that was resistant to dihydropyridine (I(VDDR)) displayed kinetics, ion selectivity and pharmacology that differed from dihydropyridine-sensitive Ca2+ currents. I(VDDR) was increased by elevating extracellular Ca2+ from 2 to 10 mM, and this caused a +30 mV shift in reversal potential. I(VDDR) was blocked by Ni2+ (100 microM) or mebefradil (1 microM) but was not affected by blockers of N-, P- or Q-type Ca2+ channels. Equimolar replacement of Ca2+ with Ba2+ reduced I(VDDR) without effects on inactivation kinetics. BayK8644 had significantly less effect on I(VDDR) than on I(VDIC). In summary, two components of inward Ca2+ current were resolved in ICC of murine small intestine and colon. Since slow waves persist in the presence of dihydropyridines, the dyhydropyridine-resistant component of inward current may contribute to slow wave propagation.
Figures





References
-
- Arnoult C, Villaz M, Florman HM. Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Molecular Pharmacology. 1998;53:1104–1111. - PubMed
-
- Bauer AJ, Publicover NG, Sanders KM. Origin and spread of slow waves in canine gastric antral circular muscle. American Journal of Physiology. 1985;249:G800–806. - PubMed
-
- Christensen J, Hauser RL. Circumferential coupling of electric slow waves in circular muscle of cat colon. American Journal of Physiology. 1971;221:1033–1037. - PubMed
-
- Ertel SI, Ertel EA. Low-voltage-activated T-type Ca2+ channels. Trends in Pharmological Sciences. 1997;18:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

