Photolysis of caged cyclic AMP in the ciliary cytoplasm of the newt olfactory receptor cell
- PMID: 12068043
- PMCID: PMC2290348
- DOI: 10.1113/jphysiol.2002.016600
Photolysis of caged cyclic AMP in the ciliary cytoplasm of the newt olfactory receptor cell
Abstract
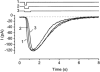
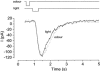
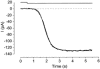
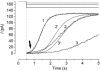
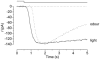
The effects of cyclic nucleotide monophosphate (cNMP) in the ciliary cytoplasm of the olfactory receptor cell were examined by using photolysis of caged cNMP loaded from the whole-cell patch clamp pipette. Illumination of the cilia induced an inward current at -50 mV. The current amplitude was voltage dependent and the polarity was reversed at +10 mV. The amplitude of the light-induced current was dependent on both light intensity and duration. The intensity-response relation was fitted well by the Hill equation with a coefficient (n(H)) of 4.99 +/- 2.66 (mean +/- S.D., n = 19) and the duration-response relation with a coefficient of 4.03 +/- 1.43 (n = 17). The activation time course of adenylyl cyclase was estimated by comparing the light-induced response with the odorant-induced response. Adenylyl cyclase was activated approximately 260 ms later from the onset of the odorant-stimulation. The light-induced current developed very sharply. This could be explained by the sequential openings of cAMP-gated and Ca2+-activated Cl- channels. At +100 mV, where Ca2+ influx is expected to be very small, the current rising phase became less steep. When the cells were stimulated by long steps of either odour or light, the odorant-induced current showed stronger decay than the light-induced response. This observation suggests that the molecular system regulating desensitization is situated upstream of cAMP production.
Figures
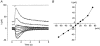

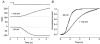
References
-
- Bakalyar HA, Reed RR. The second messenger cascade in olfactory receptor neurons. Current Opinion in Neurobiology. 1991;1:204–208. - PubMed
-
- Breer H, Boekhoff I. Second messenger signaling in olfaction. Current Opinion in Neurobiology. 1992;2:439–443. - PubMed
-
- Chen T-Y, Yau K-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

