Role of 5'AMP-activated protein kinase in glycogen synthase activity and glucose utilization: insights from patients with McArdle's disease
- PMID: 12068056
- PMCID: PMC2290379
- DOI: 10.1113/jphysiol.2002.018044
Role of 5'AMP-activated protein kinase in glycogen synthase activity and glucose utilization: insights from patients with McArdle's disease
Abstract
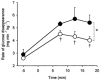
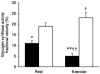
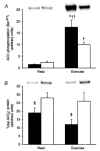
It has been suggested that 5'AMP-activated protein kinase (AMPK) is involved in the regulation of glucose and glycogen metabolism in skeletal muscle. We used patients with chronic high muscle glycogen stores and deficient glycogenolysis (McArdle's disease) as a model to address this issue. Six McArdle patients were compared with control subjects during exercise. Muscle alpha2AMPK activity increased in McArdle patients (from 1.3 +/- 0.2 to 1.9 +/- 0.2 pmol min(-1) mg(-1), P = 0.05) but not in control subjects (from 1.0 +/- 0.1 to 1.3 +/- 0.3 pmol min(-1) mg(-1)). Exercise-induced phosphorylation of the in vivo AMPK substrate acetyl CoA carboxylase (ACCbeta; Ser(221)) was higher (P < 0.01) in McArdle patients than in control subjects (18 +/- 3 vs. 10 +/- 1 arbitrary units). Exercise-induced whole-body glucose utilization was also higher in McArdle patients than in control subjects (P < 0.05). No correlation between individual AMPK or ACCbeta values and glucose utilization was observed. Glycogen synthase (GS) activity was decreased in McArdle patients from 11 +/- 1.3 to 5 +/- 1.2 % (P < 0.05) and increased in control subjects from 19 +/- 1.6 to 23 +/- 2.3 % (P < 0.05) in response to exercise. This was not associated with activity changes of GS kinase 3 or protein phosphatase 1, but the changes in GS activity could be due to changes in activity of AMPK or protein kinase A (PKA) as a negative correlation between either ACCbeta phosphorylation (Ser(221)) or plasma adrenaline and GS activity was observed. These findings suggest that GS activity is increased by glycogen breakdown and decreased by AMPK and possibly PKA activation and that the resultant GS activity depends on the relative strengths of the various stimuli. Furthermore, AMPK may be involved in the regulation of glucose utilization during exercise in humans, although the lack of correlation between individual AMPK activity or ACCbeta phosphorylation (Ser(221)) values and individual glucose utilization during exercise implies that AMPK may not be an essential regulator.
Figures


References
-
- Aschenbach WG, Suzuki Y, Breeden K, Prats C, Hirshman MF, Dufresne SD, Sakamoto K, Vilardo PG, Steele M, Kim JH, Jing SS, Goodyear LJ, Depaoli-Roach AA. The muscle-specific protein phosphatase PP1G/RGL (GM) is essential for activation of glycogen synthase by exercise. Journal of Biological Chemistry. 2001;276:39959–39967. - PubMed
-
- Bak JF, Pedersen O. Exercise-enhanced activation of glycogen synthase in human skeletal muscle. American Journal of Physiology. 1990;258:E957–963. - PubMed
-
- Begum N. Stimulation of protein phosphatase-1 activity by insulin in rat adipocytes. Evaluation of the role of mitogen-activated protein kinase pathway. Journal of Biological Chemistry. 1995;270:709–714. - PubMed
-
- Bergeron R, Russell RR, III, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. American Journal of Physiology. 1999;276:E938–944. - PubMed
-
- Bier DM, Leake RD, Haymond MW, Arnold KJ, Gruenke LD, Sperling MA, Kipnis DM. Measurement of ‘true’ glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977;26:1016–1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

