Regulation of CLV3 expression by two homeobox genes in Arabidopsis
- PMID: 12068101
- PMCID: PMC161677
- DOI: 10.1104/pp.001867
Regulation of CLV3 expression by two homeobox genes in Arabidopsis
Abstract
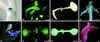
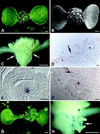
The ability of meristems to continuously produce new organs depends on the activity of their stem cell populations, which are located at the meristem tip. In Arabidopsis, the size of the stem cell domain is regulated by two antagonistic activities. The WUS (WUSCHEL) gene, encoding a homeodomain protein, promotes the formation and maintenance of stem cells. These stem cells express CLV3 (CLAVATA3), and signaling of CLV3 through the CLV1/CLV2 receptor complex restricts WUS activity. Homeostasis of the stem cell population may be achieved through feedback regulation, whereby changes in stem cell number result in corresponding changes in CLV3 expression levels, and adjustment of WUS expression via the CLV signal transduction pathway. We have analyzed whether expression of CLV3 is controlled by the activity of WUS or another homeobox gene, STM (SHOOT MERISTEMLESS), which is required for stem cell maintenance. We found that expression of CLV3 depends on WUS function only in the embryonic shoot meristem. At later developmental stages, WUS promotes the level of CLV3 expression, together with STM. Within a meristem, competence to respond to WUS activity by expressing CLV3 is restricted to the meristem apex.
Figures


References
-
- Barton MK, Poethig RS. Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wildtype and in the shoot meristemless mutant. Development. 1993;119:823–831.
-
- Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science. 2000;289:617–619. - PubMed
-
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. - PubMed
-
- Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 1996;10:967–979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

