Metabolic and environmental regulation of 3-methylcrotonyl-coenzyme A carboxylase expression in Arabidopsis
- PMID: 12068107
- PMCID: PMC161689
- DOI: 10.1104/pp.001842
Metabolic and environmental regulation of 3-methylcrotonyl-coenzyme A carboxylase expression in Arabidopsis
Abstract
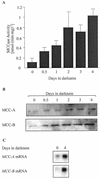
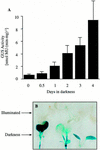
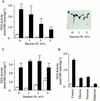
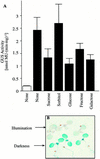
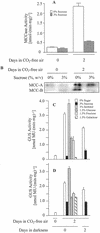
3-Methylcrotonyl-coenzyme A carboxylase (MCCase) is a nuclear-encoded, mitochondrial biotin-containing enzyme composed of two types of subunits: the biotinylated MCC-A subunit (encoded by the gene At1g03090) and the non-biotinylated MCC-B subunit (encoded by the gene At4g34030). The major metabolic role of MCCase is in the mitochondrial catabolism of leucine, and it also might function in the catabolism of isoprenoids and the mevalonate shunt. In the work presented herein, the single-copy gene encoding the Arabidopsis MCC-A subunit was isolated and characterized. It contains 15 exons separated by 14 introns. We examined the expression of the single-copy MCC-A and MCC-B genes in Arabidopsis by monitoring the accumulation of the two protein and mRNA products. In addition, the expression of these two genes was studied in transgenic plants containing the 1.1- and 1.0-kb 5' upstream sequences of the two MCCase subunit genes, respectively, fused to the beta-glucuronidase gene. Light deprivation induces MCCase expression, which is suppressed by exogenous carbohydrates, especially sucrose. Several lines of evidence indicate that the suppressor of MCCase expression is synthesized in illuminated photosynthetic organs, and can be translocated to other organs to regulate MCCase expression. These results are consistent with the hypothesis that the suppressor of MCCase expression is a carbohydrate, perhaps sucrose or a carbohydrate metabolite. We conclude that MCCase expression is primarily controlled at the level of gene transcription and regulated by a complex interplay between environmental and metabolic signals. The observed expression patterns may indicate that one of the physiological roles of MCCase is to maintain the carbon status of the organism, possibly via the catabolism of leucine.
Figures


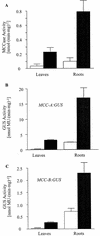
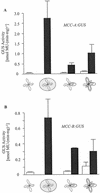
 ) or
illuminated (□) second pair of leaves. The data are means ±
) or
illuminated (□) second pair of leaves. The data are means ±

References
-
- Aubert S, Alban C, Bligny R, Douce R. Induction of beta-methylcrotonyl-coenzyme A carboxylase in higher plant cells during carbohydrate starvation: evidence for a role of MCCase in leucine catabolism. FEBS Lett. 1996;383:175–180. - PubMed
-
- Bach T. Synthesis and metabolism of mevalonic in plants. Plant Physiol Biochem. 1987;25:163–178.
-
- Bach T. Some new aspects of isoprenoid biosynthesis in plants: a review. Lipid. 1995;30:191–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

