Cytokinesis-defective mutants of Arabidopsis
- PMID: 12068111
- PMCID: PMC161693
- DOI: 10.1104/pp.004184
Cytokinesis-defective mutants of Arabidopsis
Abstract
We have identified mutations in six previously uncharacterized genes of Arabidopsis, named club, bublina, massue, rod, bloated, and bims, that are required for cytokinesis. The mutants are seedling lethal, have morphological abnormalities, and are characterized by cell wall stubs, gapped walls, and multinucleate cells. In these and other respects, the new mutants are phenotypically similar to knolle, keule, hinkel, and pleiade mutants. The mutants display a gradient of stomatal phenotypes, correlating roughly with the severity of their cytokinesis defect. Similarly, the extent to which the different mutant lines were capable of growing in tissue culture correlated well with the severity of the cytokinesis defect. Phenotypic analysis of the novel and previously characterized loci indicated that the secondary consequences of a primary defect in cytokinesis include anomalies in body organization, organ number, and cellular differentiation, as well as organ fusions and perturbations of the nuclear cycle. Two of the 10 loci are required for both cytokinesis and root hair morphogenesis. The results have implications for the identification of novel cytokinesis genes and highlight the mechanistic similarity between cytokinesis and root hair morphogenesis, two processes that result in a rapid deposition of new cell walls via polarized secretion.
Figures


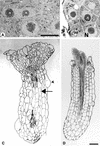
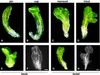


References
-
- Assaad FF. Of weeds and men: what genomes teach us about plant cell biology. Curr Opin Plant Biol. 2001b;4:478–487. - PubMed
-
- Assaad FF, Mayer U, Wanner G, Jürgens G. The KEULE gene is involved in cytokinesis in Arabidopsis. Mol Gen Genet. 1996;253:267–277. - PubMed
-
- Balasubramanian MK, McCollum D, Surana U. Tying the knot: linking cytokinesis to the nuclear cycle. J Cell Sci. 2000;113:1503–1513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

