Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii
- PMID: 12068117
- PMCID: PMC161699
- DOI: 10.1104/pp.002410
Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii
Abstract
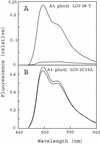
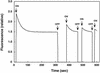
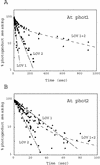
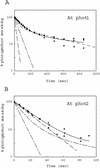
Phototropins (phot1 and phot2, formerly designated nph1 and npl1) are blue-light receptors that mediate phototropism, blue light-induced chloroplast relocation, and blue light-induced stomatal opening in Arabidopsis. Phototropins contain two light, oxygen, or voltage (LOV) domains at their N termini (LOV1 and LOV2), each a binding site for the chromophore flavin mononucleotide (FMN). Their C termini contain a serine/threonine protein kinase domain. Here, we examine the kinetic properties of the LOV domains of Arabidopsis phot1 and phot2, rice (Oryza sativa) phot1 and phot2, and Chlamydomonas reinhardtii phot. When expressed in Escherichia coli, purified LOV domains from all phototropins examined bind FMN tightly and undergo a self-contained photocycle, characterized by fluorescence and absorption changes induced by blue light (T. Sakai, T. Kagawa, M. Kasahara, T.E. Swartz, J.M. Christie, W.R. Briggs, M. Wada, K. Okada [2001] Proc Natl Acad Sci USA 98: 6969-6974; M. Salomon, J.M. Christie, E. Knieb, U. Lempert, W.R. Briggs [2000] Biochemistry 39: 9401-9410). The photocycle involves the light-induced formation of a cysteinyl adduct to the C(4a) carbon of the FMN chromophore, which subsequently breaks down in darkness. In each case, the relative quantum efficiencies for the photoreaction and the rate constants for dark recovery of LOV1, LOV2, and peptides containing both LOV domains are presented. Moreover, the data obtained from full-length Arabidopsis phot1 and phot2 expressed in insect cells closely resemble those obtained for the tandem LOV-domain fusion proteins expressed in E. coli. For both Arabidopsis and rice phototropins, the LOV domains of phot1 differ from those of phot2 in their reaction kinetic properties and relative quantum efficiencies. Thus, in addition to differing in amino acid sequence, the phototropins can be distinguished on the basis of the photochemical cycles of their LOV domains. The LOV domains of C. reinhardtii phot also undergo light-activated spectral changes consistent with cysteinyl adduct formation. Thus, the phototropin family extends over a wide evolutionary range from unicellular algae to higher plants.
Figures



References
-
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. - PubMed
-
- Briggs WR, Christie JM, Salomon M. Phototropins: a new family of flavin-binding blue light receptors in plants. Antioxidants Redox Signaling. 2001b;3:775–788. - PubMed
-
- Briggs WR, Huala E. Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol. 1999;15:33–62. - PubMed
-
- Christie JM, Briggs WR. Blue light sensing in higher plants. J Biol Chem. 2001;276:11457–11460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

