Functional analysis of the cellulose synthase genes CesA1, CesA2, and CesA3 in Arabidopsis
- PMID: 12068120
- PMCID: PMC161702
- DOI: 10.1104/pp.010931
Functional analysis of the cellulose synthase genes CesA1, CesA2, and CesA3 in Arabidopsis
Abstract
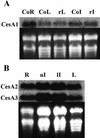
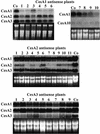
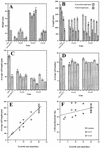
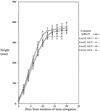
Polysaccharide analyses of mutants link several of the glycosyltransferases encoded by the 10 CesA genes of Arabidopsis to cellulose synthesis. Features of those mutant phenotypes point to particular genes depositing cellulose predominantly in either primary or secondary walls. We used transformation with antisense constructs to investigate the functions of CesA2 (AthA) and CesA3 (AthB), genes for which reduced synthesis mutants are not yet available. Plants expressing antisense CesA1 (RSW1) provided a comparison with a gene whose mutant phenotype (Rsw1(-)) points mainly to a primary wall role. The antisense phenotypes of CesA1 and CesA3 were closely similar and correlated with reduced expression of the target gene. Reductions in cell length rather than cell number underlay the shorter bolts and stamen filaments. Surprisingly, seedling roots were unaffected in both CesA1 and CesA3 antisense plants. In keeping with the mild phenotype compared with Rsw1(-), reductions in total cellulose levels in antisense CesA1 and CesA3 plants were at the borderline of significance. We conclude that CesA3, like CesA1, is required for deposition of primary wall cellulose. To test whether there were important functional differences between the two, we overexpressed CesA3 in rsw1 but were unable to complement that mutant's defect in CesA1. The function of CesA2 was less obvious, but, consistent with a role in primary wall deposition, the rate of stem elongation was reduced in antisense plants growing rapidly at 31 degrees C.
Figures




References
-
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science. 1998;279:717–720. - PubMed
-
- Baskin TI, Betzner AS, Hoggart R, Cork A, Williamson RE. Root morphology mutants in Arabidopsis thaliana. Aust J Plant Physiol. 1992;19:427–437.
-
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana. C R Acad Sci Ser III Sci Vie. 1993;316:1194–1199.
-
- Brown RM, Jr, Saxena IM, Kudlicka K. Cellulose biosynthesis in higher plants. Trends Plant Sci. 1996;1:149–156.
-
- Datla RSS, Hammerlindl JK, Panchuk B, Pelcher LE, Keller W. Modified binary plant transformation vectors with the wild-type gene encoding NPTII. Gene. 1992;211:383–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

