Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis
- PMID: 12068123
- PMCID: PMC161705
- DOI: 10.1104/pp.001362
Heat stress- and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis
Abstract
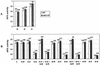
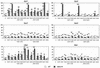
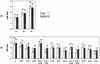
To find evidence for a connection between heat stress response, oxidative stress, and common stress tolerance, we studied the effects of elevated growth temperatures and heat stress on the activity and expression of ascorbate peroxidase (APX). We compared wild-type Arabidopsis with transgenic plants overexpressing heat shock transcription factor 3 (HSF3), which synthesize heat shock proteins and are improved in basal thermotolerance. Following heat stress, APX activity was positively affected in transgenic plants and correlated with a new thermostable isoform, APX(S). This enzyme was present in addition to thermolabile cytosolic APX1, the prevalent isoform in unstressed cells. In HSF3-transgenic plants, APX(S) activity was detectable at normal temperature and persisted after severe heat stress at 44 degrees C. In nontransgenic plants, APX(S) was undetectable at normal temperature, but could be induced by moderate heat stress. The mRNA expression profiles of known and three new Apx genes were determined using real-time PCR. Apx1 and Apx2 genes encoding cytosolic APX were heat stress and HSF dependently expressed, but only the representations of Apx2 mRNA met the criteria that suggest identity between APX(S) and APX2: not expressed at normal temperature in wild type, strong induction by heat stress, and HSF3-dependent expression in transgenic plants. Our data suggest that Apx2 is a novel heat shock gene and that the enzymatic activity of APX2/APX(S) is required to compensate heat stress-dependent decline of APX1 activity in the cytosol. The functional roles of modulations of APX expression and the interdependence of heat stress and oxidative stress response and signaling mechanisms are discussed.
Figures





References
-
- Amako K, Chen G, Asada K. Separate assays for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504.
-
- An Y-Q, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 1996;10:107–121. - PubMed
-
- Banzet N, Richaud C, Deveaux Y, Kazmaier M, Gagnon J, Triantaphlides C. Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. Plant J. 1998;13:519–527. - PubMed
-
- Barros MD, Czarnecka E, Gurley WB. Mutational analysis of a plant heat shock element. Plant Mol Biol. 1992;19:665–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

