Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials
- PMID: 12069980
- PMCID: PMC127288
- DOI: 10.1128/AAC.46.7.2238-2243.2002
Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials
Abstract
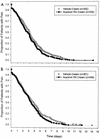
Acyclovir cream has been available for the treatment of herpes labialis in numerous countries outside the United States for over a decade. Evidence for its efficacy comes from a few small clinical trials conducted in the 1980s. To examine more comprehensively the efficacy and safety of this formulation, we conducted two independent, identical, parallel, randomized, double-blind, vehicle-controlled, large-scale multicenter clinical trials. Healthy adults with a history of frequent herpes labialis were recruited from the general population, screened for eligibility, randomized equally to 5% acyclovir cream or vehicle control, given study medication, and told to self-initiate treatment five times daily for 4 days beginning within 1 h of the onset of a recurrent episode. The number of patients who treated a lesion was 686 in study 1 and 699 in study 2. In study 1, the mean duration of episodes was 4.3 days for patients treated with acyclovir cream and 4.8 days for those treated with the vehicle control (hazards ratio [HR] = 1.23; 95% confidence interval [CI], 1.06 to 1.44; P = 0.007). In study 2, the mean duration of episodes was 4.6 days for patients treated with acyclovir cream and 5.2 days for those treated with the vehicle control (HR = 1.24; 95% CI, 1.06 to 1.44; P = 0.006). Efficacy was apparent whether therapy was initiated "early" (prodrome or erythema lesion stage) or "late" (papule or vesicle stage). There was a statistically significant reduction in the duration of lesion pain in both studies. Acyclovir cream did not prevent the development of classical lesions (progression to vesicles, ulcers, and/or crusts). Adverse events were mild and infrequent.
Figures


Similar articles
-
Penciclovir cream for the treatment of herpes simplex labialis. A randomized, multicenter, double-blind, placebo-controlled trial. Topical Penciclovir Collaborative Study Group.JAMA. 1997 May 7;277(17):1374-9. JAMA. 1997. PMID: 9134943 Clinical Trial.
-
Penciclovir cream for the treatment of sunlight-induced herpes simplex labialis: a randomized, double-blind, placebo-controlled trial. Penciclovir Cream Herpes Labialis Study Group.Clin Ther. 2000 Jan;22(1):76-90. doi: 10.1016/s0149-2918(00)87979-5. Clin Ther. 2000. PMID: 10688392 Clinical Trial.
-
Effective treatment of herpes simplex labialis with penciclovir cream: combined results of two trials.J Am Dent Assoc. 2002 Mar;133(3):303-9. doi: 10.14219/jada.archive.2002.0169. J Am Dent Assoc. 2002. PMID: 11934185 Clinical Trial.
-
Acyclovir in the management of herpes labialis.J Antimicrob Chemother. 1983 Sep;12 Suppl B:95-103. doi: 10.1093/jac/12.suppl_b.95. J Antimicrob Chemother. 1983. PMID: 6355056 Review.
-
The role of topical 5% acyclovir and 1% hydrocortisone cream (Xerese™) in the treatment of recurrent herpes simplex labialis.Postgrad Med. 2010 Sep;122(5):1-6. doi: 10.3810/pgm.2010.09.2216. Postgrad Med. 2010. PMID: 20873400 Review.
Cited by
-
Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: a randomised controlled trial.BMJ Open. 2019 May 14;9(5):e026201. doi: 10.1136/bmjopen-2018-026201. BMJ Open. 2019. PMID: 31092654 Free PMC article. Clinical Trial.
-
Viral infections of oral cavity.J Family Med Prim Care. 2020 Jan 28;9(1):36-42. doi: 10.4103/jfmpc.jfmpc_807_19. eCollection 2020 Jan. J Family Med Prim Care. 2020. PMID: 32110562 Free PMC article. Review.
-
The diagnosis and management of oral herpes simplex infection.Curr Infect Dis Rep. 2006 May;8(3):181-8. doi: 10.1007/s11908-006-0057-x. Curr Infect Dis Rep. 2006. PMID: 16643769
-
Shallominthe active antimicrobial constituent of persian shallot in treatment of oral herpes: a double-blind randomized clinical trial.Jundishapur J Nat Pharm Prod. 2014 Aug 1;9(3):e17372. doi: 10.17795/jjnpp-17372. eCollection 2014 Aug. Jundishapur J Nat Pharm Prod. 2014. PMID: 25237646 Free PMC article.
-
Cyclodextrins in the antiviral therapy.J Drug Deliv Sci Technol. 2021 Aug;64:102589. doi: 10.1016/j.jddst.2021.102589. Epub 2021 May 20. J Drug Deliv Sci Technol. 2021. PMID: 34035845 Free PMC article. Review.
References
-
- Fiddian, A. P., and L. Ivanyi. 1983. Topical acyclovir in the management of recurrent herpes labialis. Br. J. Dermatol. 109:321-326. - PubMed
-
- Green, J. A., S. L. Spruance, G. Wenerstrom, and M. W. Piepkorn. 1985. Post-herpetic erythema multiforme prevented with prophylactic oral acyclovir. Ann. Intern. Med. 102:632-633. - PubMed
-
- Harris, E. K., and A. Albert. 1991. Survivorship analysis for clinical studies. Marcel Dekker, New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

