Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells
- PMID: 12070279
- PMCID: PMC2193554
- DOI: 10.1084/jem.20020033
Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells
Abstract
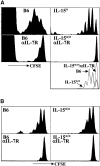
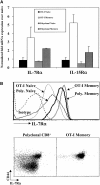
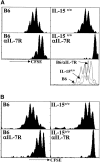
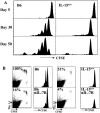
Both naive and memory T cells undergo antigen-independent proliferation after transfer into a T cell-depleted environment (acute homeostatic proliferation), whereas only memory T cells slowly divide in a full T cell compartment (basal proliferation). We show, first, that naive and memory CD8+ T cells have different cytokine requirements for acute homeostatic proliferation. Interleukin (IL)-7 receptor(R)alpha-mediated signals were obligatory for proliferation of naive T cells in lymphopenic hosts, whereas IL-15 did not influence their division. Memory T cells, on the other hand, could use either IL-7Ralpha- or IL-15-mediated signals for acute homeostatic proliferation: their proliferation was delayed when either IL-7Ralpha was blocked or IL-15 removed, but only when both signals were absent was proliferation ablated. Second, the cytokine requirements for basal and acute homeostatic proliferation of CD8+ memory T cells differ, as basal division of memory T cells was blocked completely in IL-15-deficient hosts. These data suggest a possible mechanism for the dearth of memory CD8+ T cells in IL-15- and IL-15Ralpha-deficient mice is their impaired basal proliferation. Our results show that naive and memory T lymphocytes differ in their cytokine dependence for acute homeostatic proliferation and that memory T lymphocytes have distinct requirements for proliferation in full versus empty compartments.
Figures
Comment in
-
Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15.J Exp Med. 2002 Jun 17;195(12):F49-52. doi: 10.1084/jem.20020767. J Exp Med. 2002. PMID: 12070294 Free PMC article. No abstract available.
References
-
- Marrack, P., J. Bender, D. Hildeman, M. Jordan, T. Mitchell, M. Murakami, A. Sakamoto, B.C. Schaefer, B. Swanson, and J. Kappler. 2000. Homeostasis of αβ TCR+ T cells. Nat. Immunol. 1:107–111. - PubMed
-
- Freitas, A.A., and B. Rocha. 2000. Population biology of lymphocytes: the fight for survival. Annu. Rev. Immunol. 18:83–111. - PubMed
-
- Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. - PubMed
-
- Murali-Krishna, K., L.L. Ming, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I deficient mice. Science. 286:1377–1381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

