Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation
- PMID: 12070283
- PMCID: PMC2193567
- DOI: 10.1084/jem.20011700
Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation
Abstract
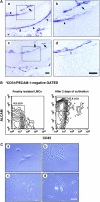
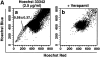
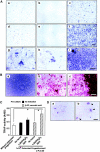
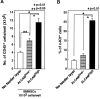
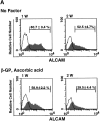
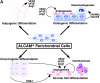
Perichondrium in fetal limb is composed of undifferentiated mesenchymal cells. However, the multipotency of cells in this region and the role of perichondrium in bone marrow formation are not well understood. In this report, we purified and characterized perichondrial cells using a monoclonal antibody against activated leukocyte cell adhesion molecule (ALCAM) and investigated the role of perichondrial cells in hematopoietic bone marrow formation. ALCAM is expressed on hematopoietic cells, endothelial cells, bone marrow stromal cells, and mesenchymal stem cells and mediates homophilic (ALCAM-ALCAM)/heterophilic (ALCAM-CD6) cell adhesion. Here we show by immunohistochemical staining that ALCAM is expressed in perichondrium. ALCAM+ perichondrial cells isolated by FACS exhibit the characteristics of mesenchymal stem cells. ALCAM+ cells can differentiate into osteoblasts, adipocytes, chondrocytes, and stromal cells, which can support osteoclastogenesis, hematopoiesis, and angiogenesis. Furthermore, the addition of ALCAM-Fc or CD6-Fc to the metatarsal culture, the invasion of the blood vessels to a cartilage was inhibited. Our findings indicate that ALCAM+ perichondrial cells participate in vascular invasion by recruiting osteoclasts and vessels. These findings suggest that perichondrium might serve as a stem cell reservoir and play an important role in the early development of a bone and bone marrow.
Figures











References
-
- Hinchcliffe, J.R., and D.R. Johnson. 1990. The development of the vertebrate limb. Clarendon Press, Oxford. 1–300.
-
- Horton, W.A. 1993. Cartilage Morphology. Extracellular Matrix and Heritable Disorders of Connective Tissue. P.M. Royce and B. Steinman, editors. Alan R. Liss, New York. pp. 73–84.
-
- Caplan, A.I., and D.G. Pechak. 1987. The cellular and molecular embryology of bone formation. Bone and Mineral Research. W.A. Peck, editor, Elsevier, New York. pp. 117–183.
-
- Baron, R.E. 1996. Anatomy and ultrastructure of bone. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, third edition. M.J. Favus, editor, Lippincott-Raven, New York. pp. 3–10.
-
- Pittenger, M.F., A.M. Mackay, S.C. Beck, R.K. Jaiswal, R. Douglas, J.D. Mosca, M.A. Moorman, D.W. Simonetti, S. Creig, and D.R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

