Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence
- PMID: 12070289
- PMCID: PMC2193562
- DOI: 10.1084/jem.20020258
Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence
Abstract
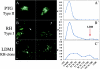
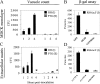
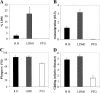
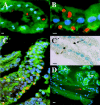
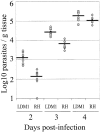
After oral ingestion, Toxoplasma gondii crosses the intestinal epithelium, disseminates into the deep tissues, and traverses biological barriers such as the placenta and the blood-brain barrier to reach sites where it causes severe pathology. To examine the cellular basis of these processes, migration of T. gondii was studied in vitro using polarized host cell monolayers and extracellular matrix. Transmigration required active parasite motility and the highly virulent type I strains consistently exhibited a superior migratory capacity than the nonvirulent type II and type III strains. Type I strain parasites also demonstrated a greater capacity for transmigration across mouse intestine ex vivo, and directly penetrated into the lamina propria and vascular endothelium. A subpopulation of virulent type I parasites exhibited a long distance migration (LDM) phenotype in vitro, that was not expressed by nonvirulent type II and type III strains. Cloning of parasites expressing the LDM phenotype resulted in substantial increase of migratory capacity in vitro and in vivo. The potential to up-regulate migratory capacity in T. gondii likely plays an important role in establishing new infections and in dissemination upon reactivation of chronic infections.
Figures
References
-
- Desmonts, G., and J. Couvreur. 1974. Congenital toxoplasmosis: a prospective study of 378 pregnancies. N. Engl. J. Med. 290:1110–1116. - PubMed
-
- Luft, B.J., R. Hafner, A.H. Korzun, C. Leport, D. Antoniskis, E.M. Bosler, D.D. Bourland, R. Uttamchandani, J. Fuhrer, J. Jacobson, et al. 1993. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 329:995–1000. - PubMed
-
- Roberts, F., and R. McLeod. 1999. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol. Today. 15:51–57. - PubMed
-
- Howe, D.K., and L.D. Sibley. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172:1561–1566. - PubMed

