Identification of regulatory T cells in tolerated allografts
- PMID: 12070291
- PMCID: PMC2193557
- DOI: 10.1084/jem.20012097
Identification of regulatory T cells in tolerated allografts
Abstract
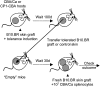
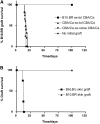
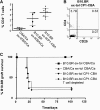
Induction of transplantation tolerance with certain therapeutic nondepleting monoclonal antibodies can lead to a robust state of peripheral "dominant" tolerance. Regulatory CD4+ T cells, which mediate this form of "dominant" tolerance, can be isolated from spleens of tolerant animals. To determine whether there were any extra-lymphoid sites that might harbor regulatory T cells we sought their presence in tolerated skin allografts and in normal skin. When tolerated skin grafts are retransplanted onto T cell-depleted hosts, graft-infiltrating T cells exit the graft and recolonize the new host. These colonizing T cells can be shown to contain members with regulatory function, as they can prevent nontolerant lymphocytes from rejecting fresh skin allografts, without hindrance of rejection of third party skin. Our results suggest that T cell suppression of graft rejection is an active process that operates beyond secondary lymphoid tissue, and involves the persistent presence of regulatory T cells at the site of the tolerated transplant.
Figures


Similar articles
-
Linked suppression of skin graft rejection can operate through indirect recognition.J Immunol. 1998 Dec 1;161(11):5813-6. J Immunol. 1998. PMID: 9834057
-
Alloantigen-induced, T-cell-dependent production of nitric oxide by macrophages infiltrating skin allografts in mice.Transpl Int. 2002 Mar;15(2-3):108-16. doi: 10.1007/s00147-002-0378-0. Epub 2002 Mar 1. Transpl Int. 2002. PMID: 11935167
-
Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells.Eur J Immunol. 1994 Oct;24(10):2383-92. doi: 10.1002/eji.1830241019. Eur J Immunol. 1994. PMID: 7925565
-
Lymphocytes selected in allogeneic thymic epithelium mediate dominant tolerance toward tissue grafts of the thymic epithelium haplotype.Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7555-9. doi: 10.1073/pnas.92.16.7555. Proc Natl Acad Sci U S A. 1995. PMID: 7638230 Free PMC article.
-
Organ transplant specificity of tolerance to skin grafts with heart or kidney grafts plus nondepleting anti-CD4 monoclonal antibody (RIB 5/2) and intravenous donor alloantigen administration.J Surg Res. 2001 Jun 1;98(1):59-65. doi: 10.1006/jsre.2001.6169. J Surg Res. 2001. PMID: 11368539
Cited by
-
T helper cell mediated-tolerance towards fetal allograft in successful pregnancy.Clin Mol Allergy. 2015 Jun 10;13(1):9. doi: 10.1186/s12948-015-0015-y. eCollection 2015. Clin Mol Allergy. 2015. PMID: 26064081 Free PMC article.
-
Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways.Immunology. 2012 Dec;137(4):305-16. doi: 10.1111/imm.12008. Immunology. 2012. PMID: 23167249 Free PMC article.
-
Regulatory T cell modulation of cytokine and cellular networks in corneal graft rejection.Curr Ophthalmol Rep. 2018 Dec;6(4):266-274. Epub 2018 Oct 6. Curr Ophthalmol Rep. 2018. PMID: 31807370 Free PMC article.
-
CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo.J Exp Med. 2003 Jul 21;198(2):259-66. doi: 10.1084/jem.20030654. J Exp Med. 2003. PMID: 12874259 Free PMC article.
-
Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo.Blood. 2005 Nov 1;106(9):3097-104. doi: 10.1182/blood-2005-05-1864. Epub 2005 Jul 12. Blood. 2005. PMID: 16014565 Free PMC article.
References
-
- Waldmann, H. 1999. Transplantation tolerance-where do we stand? Nat. Med. 5:1245–1248. - PubMed
-
- Wekerle, T., and M. Sykes. 2001. Mixed chimerism and transplantation tolerance. Annu. Rev. Med. 52:353–370. - PubMed
-
- Knechtle, S.J. 2000. Knowledge about transplantation tolerance gained in primates. Curr. Opin. Immunol. 12:552–556. - PubMed
-
- Kirk, A.D., and D.M. Harlan. 2000. Challenges for the clinical application of transplant tolerance strategies. Curr. Opin. Organ Transplant. 5:108–113.
-
- Li, X.C., T.B. Strom, L.A. Turka, and A.D. Wells. 2001. T cell death and transplantation tolerance. Immunity. 14:407–416. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

