Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases
- PMID: 12070302
- PMCID: PMC151012
- DOI: 10.1172/JCI15234
Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases
Abstract
TGF-betas are potent inhibitors of epithelial cell proliferation. However, in established carcinomas, autocrine/paracrine TGF-beta interactions can enhance tumor cell viability and progression. Thus, we studied the effect of a soluble Fc:TGF-beta type II receptor fusion protein (Fc:TbetaRII) on transgenic and transplantable models of breast cancer metastases. Systemic administration of Fc:TbetaRII did not alter primary mammary tumor latency in MMTV-Polyomavirus middle T antigen transgenic mice. However, Fc:TbetaRII increased apoptosis in primary tumors, while reducing tumor cell motility, intravasation, and lung metastases. These effects correlated with inhibition of Akt activity and FKHRL1 phosphorylation. Fc:TbetaRII also inhibited metastases from transplanted 4T1 and EMT-6 mammary tumors in syngeneic BALB/c mice. Tumor microvessel density in a mouse dorsal skin window chamber was unaffected by Fc:TbetaRII. Therefore, blockade of TGF-beta signaling may reduce tumor cell viability and migratory potential and represents a testable therapeutic approach against metastatic carcinomas.
Figures



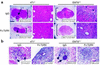
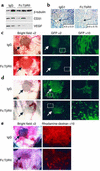
Comment in
-
TGF-beta antagonists: why suppress a tumor suppressor?J Clin Invest. 2002 Jun;109(12):1533-6. doi: 10.1172/JCI15970. J Clin Invest. 2002. PMID: 12070299 Free PMC article. Review. No abstract available.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. - PubMed
-
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. - PubMed
-
- Pierce DF, Jr, et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 1993;7:2308–2317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

