Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent
- PMID: 12070304
- PMCID: PMC151009
- DOI: 10.1172/JCI14308
Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent
Abstract
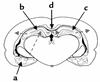
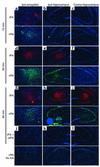
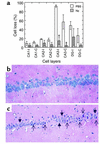
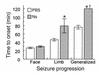
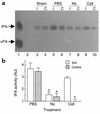
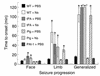
Tissue-type plasminogen activator (tPA) is a highly specific serine proteinase expressed in the CNS during events that require neuronal plasticity. In this study we demonstrate that endogenous tPA mediates the progression of kainic acid-induced (KA-induced) seizures by promoting the synchronization of neuronal activity required for seizure spreading, and that, unlike KA-induced cell death, this activity is plasminogen-independent. Specifically, seizure induction by KA injection into the amygdala induces tPA activity and cell death in both hippocampi, and unilateral treatment of rats with neuroserpin, a natural inhibitor of tPA in the brain, enhances neuronal survival in both hippocampi. Inhibition of tPA within the hippocampus by neuroserpin treatment does not prevent seizure onset but instead markedly delays the progression of seizure activity in both rats and wild-type mice. In tPA-deficient mice, seizure progression is significantly delayed, and neuroserpin treatment does not further delay seizure spreading. In contrast, plasminogen-deficient mice show a pattern of seizure spreading and a response to neuroserpin that is similar to that of wild-type animals. These findings indicate that tPA acts on a substrate other than plasminogen and that the effects of neuroserpin on seizure progression and neuronal cell survival are mediated through the inhibition of tPA.
Figures

Comment in
-
Tissue plasminogen activator and seizures: a clot-buster's secret life.J Clin Invest. 2002 Jun;109(12):1529-31. doi: 10.1172/JCI15961. J Clin Invest. 2002. PMID: 12070298 Free PMC article. Review. No abstract available.
References
-
- Carroll PM, Tsirka SE, Richards WG, Frohman MA, Strickland S. The mouse tissue-plasminogen activator gene 5′ flanking region directs appropriate expression in development and a seizure-enhanced response in the CNS. Development. 1994;120:3173–3183. - PubMed
-
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. - PubMed
-
- Seeds NW, Williams BL, Bickford PC. Tissue-plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science. 1995;270:1992–1994. - PubMed
-
- Bugge TH, et al. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. - PubMed
-
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue-plasminogen activator. Nature. 1995;377:340–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

