Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S
- PMID: 12070305
- PMCID: PMC151014
- DOI: 10.1172/JCI15272
Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S
Abstract
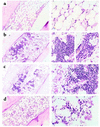
Whole-body irradiation at the minimal lethal dose causes bone marrow failure and death within 12-18 days. To identify the principal components of the hematopoietic system that are radioprotective, we transplanted lethally irradiated mice with purified progenitors: common myeloid progenitors (CMPs), megakaryocyte/erythrocyte-restricted progenitors (MEPs), or granulocyte/monocyte-restricted progenitors (GMPs). Transplanted CMPs gave rise to cells both of the granulocyte/monocyte (GM) series and the megakaryocyte/erythrocyte series, whereas GMPs or MEPs showed reconstitution of only GM or ME cells, respectively. CMPs and MEPs but not GMPs protected mice in a dose-dependent manner, suggesting that erythrocytes, platelets, or both are the critical effectors of radioprotection. Accordingly, CMPs and MEPs formed robust colonies in recipient bone marrow and spleen, whereas GMPs formed small colonies that rapidly disappeared. Direct comparisons of spleen CFU (CFU-S) potentials among each progenitor subset showed that MEPs contain the vast majority of day 8 CFU-S activity, suggesting that day 8 CFU-S are the precursors of radioprotective cell subsets. All animals radioprotected for 30 days subsequently survived for at least 6 months post-transplant, and showed only host-derived hematopoiesis after 30 days. These findings suggest that rare hematopoietic stem cells survive myeloablation that can eventually repopulate irradiated hosts if myeloerythroid-restricted progenitors transiently rescue ablated animals through the critical window of bone marrow failure.
Figures


Comment in
-
Optimizing hematopoietic recovery following bone marrow transplantation.J Clin Invest. 2002 Jun;109(12):1527-8. doi: 10.1172/JCI15916. J Clin Invest. 2002. PMID: 12070297 Free PMC article. No abstract available.
Similar articles
-
Optimizing hematopoietic recovery following bone marrow transplantation.J Clin Invest. 2002 Jun;109(12):1527-8. doi: 10.1172/JCI15916. J Clin Invest. 2002. PMID: 12070297 Free PMC article. No abstract available.
-
Sox17 as a candidate regulator of myeloid restricted differentiation potential.Dev Growth Differ. 2014 Aug;56(6):469-79. doi: 10.1111/dgd.12147. Epub 2014 Aug 5. Dev Growth Differ. 2014. PMID: 25093513
-
Dendritic cell potentials of early lymphoid and myeloid progenitors.Blood. 2001 Jun 1;97(11):3333-41. doi: 10.1182/blood.v97.11.3333. Blood. 2001. PMID: 11369621
-
CD62L expression level determines the cell fate of myeloid progenitors.Stem Cell Reports. 2021 Dec 14;16(12):2871-2886. doi: 10.1016/j.stemcr.2021.10.012. Epub 2021 Nov 18. Stem Cell Reports. 2021. PMID: 34798065 Free PMC article.
-
Role of transcription factors in differentiation and reprogramming of hematopoietic cells.Keio J Med. 2011;60(2):47-55. doi: 10.2302/kjm.60.47. Keio J Med. 2011. PMID: 21720200 Review.
Cited by
-
Identification of the minimum requirements for successful haematopoietic stem cell transplantation.Br J Haematol. 2022 Feb;196(3):711-723. doi: 10.1111/bjh.17867. Epub 2021 Dec 20. Br J Haematol. 2022. PMID: 34927242 Free PMC article.
-
Ex vivo expansion potential of murine hematopoietic stem cells is a rare property only partially predicted by phenotype.Elife. 2024 Mar 6;12:RP91826. doi: 10.7554/eLife.91826. Elife. 2024. PMID: 38446538 Free PMC article.
-
Plasticity and maintenance of hematopoietic stem cells during development.Recent Pat Biotechnol. 2011 Apr;5(1):40-53. doi: 10.2174/187220811795655896. Recent Pat Biotechnol. 2011. PMID: 21517745 Free PMC article. Review.
-
Epidermal growth factor regulates hematopoietic regeneration after radiation injury.Nat Med. 2013 Mar;19(3):295-304. doi: 10.1038/nm.3070. Epub 2013 Feb 3. Nat Med. 2013. PMID: 23377280 Free PMC article.
-
Leukemic marrow infiltration reveals a novel role for Egr3 as a potent inhibitor of normal hematopoietic stem cell proliferation.Blood. 2015 Sep 10;126(11):1302-13. doi: 10.1182/blood-2015-01-623645. Epub 2015 Jul 17. Blood. 2015. PMID: 26186938 Free PMC article.
References
-
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. - PubMed
-
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. - PubMed
-
- Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1loLin–Sca-1+hematopoietic stem cells. Blood. 1994;83:3758–3779. - PubMed
-
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. - PubMed
-
- Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

