APRIL modulates B and T cell immunity
- PMID: 12070306
- PMCID: PMC151011
- DOI: 10.1172/JCI15034
APRIL modulates B and T cell immunity
Abstract
The TNF-like ligands APRIL and BLyS are close relatives and share the capacity to bind the receptors TACI and BCMA. BLyS has been shown to play an important role in B cell homeostasis and autoimmunity, but the biological role of APRIL remains less well defined. Analysis of T cells revealed an activation-dependent increase in APRIL mRNA expression. We therefore generated mice expressing APRIL as a transgene in T cells. These mice appeared normal and showed no signs of B cell hyperplasia. Transgenic T cells revealed a greatly enhanced survival in vitro as well as enhanced survival of staphylococcal enterotoxin B-reactive CD4+ T cells in vivo, which both directly correlate with elevated Bcl-2 levels. Analysis of humoral responses to T cell-dependent antigens in the transgenic mice indicated that APRIL affects only IgM but not IgG responses. In contrast, T cell-independent type 2 (TI-2) humoral response was enhanced in APRIL transgenic mice. As TACI was previously reported to be indispensable for TI-2 antibody formation, these results suggest a role for APRIL/TACI interactions in the generation of this response. Taken together, our data indicate that APRIL is involved in the induction and/or maintenance of T and B cell responses.
Figures
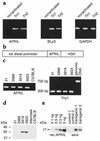
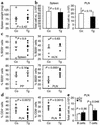
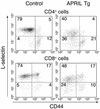





Similar articles
-
The anti-apoptotic factor Bcl-2 can functionally substitute for the B cell survival but not for the marginal zone B cell differentiation activity of BAFF.Eur J Immunol. 2004 Feb;34(2):509-18. doi: 10.1002/eji.200324692. Eur J Immunol. 2004. PMID: 14768056
-
The role of APRIL and BAFF in lymphocyte activation.Curr Opin Immunol. 2005 Jun;17(3):282-9. doi: 10.1016/j.coi.2005.04.005. Curr Opin Immunol. 2005. PMID: 15886118 Review.
-
Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA.Biochemistry. 2005 Feb 15;44(6):1919-31. doi: 10.1021/bi048227k. Biochemistry. 2005. PMID: 15697217
-
TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice.Nat Immunol. 2001 Jul;2(7):632-7. doi: 10.1038/89782. Nat Immunol. 2001. PMID: 11429548
-
The uncertain glory of APRIL.Cell Death Differ. 2003 Oct;10(10):1121-5. doi: 10.1038/sj.cdd.4401291. Cell Death Differ. 2003. PMID: 14502235 Review.
Cited by
-
Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies.Annu Rev Med. 2013;64:339-56. doi: 10.1146/annurev-med-041811-142014. Epub 2012 Oct 16. Annu Rev Med. 2013. PMID: 23072577 Free PMC article. Review.
-
Functional expression of a proliferation-related ligand in hepatocellular carcinoma and its implications for neovascularization.World J Gastroenterol. 2005 Aug 14;11(30):4650-4. doi: 10.3748/wjg.v11.i30.4650. World J Gastroenterol. 2005. PMID: 16094704 Free PMC article.
-
Delayed onset of autoreactive antibody production and M2-skewed macrophages contribute to improved survival of TACI deficient MRL-Fas/Lpr mouse.Sci Rep. 2018 Jan 22;8(1):1308. doi: 10.1038/s41598-018-19827-8. Sci Rep. 2018. PMID: 29358664 Free PMC article.
-
APRIL:TACI axis is dispensable for the immune response to rabies vaccination.Antiviral Res. 2017 Aug;144:130-137. doi: 10.1016/j.antiviral.2017.06.004. Epub 2017 Jun 12. Antiviral Res. 2017. PMID: 28619678 Free PMC article.
-
TACI-Fc gene therapy improves autoimmune sialadenitis but not salivary gland function in non-obese diabetic mice.Oral Dis. 2012 May;18(4):365-74. doi: 10.1111/j.1601-0825.2011.01885.x. Epub 2011 Dec 29. Oral Dis. 2012. PMID: 22212434 Free PMC article.
References
-
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. - PubMed
-
- Moore PA, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. - PubMed
-
- Mukhopadhyay A, Ni J, Zhai Y, Yu GL, Aggarwal BB. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J Biol Chem. 1999;274:15978–15981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

