Chemical-modification rescue assessed by mass spectrometry demonstrates that gamma-thia-lysine yields the same activity as lysine in aldolase
- PMID: 12070312
- PMCID: PMC2373652
- DOI: 10.1110/ps.3900102
Chemical-modification rescue assessed by mass spectrometry demonstrates that gamma-thia-lysine yields the same activity as lysine in aldolase
Abstract
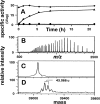
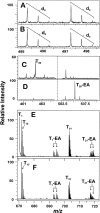
The role of active site residues in fructose 1,6-bisphosphate aldolase is investigated by chemical-modification rescue. An active-site mutation, K107C, is constructed in a background where the four solvent-accessible cysteine residues are converted to alanine. The resulting mutant, tetK107C, when reacted with bromoethylamine (BrEA), shows a 40-fold increase in activity (to 80% that of wild type). Determination of the sites and their degree of modification using electrospray ionization Fourier transform mass spectrometry (ESI-FTMS) is developed, allowing correlation of activity after chemical modification rescue to the degree of modification. The stoichiometry of the reaction is 2.5 aminoethylations per subunit, as measured by ESI-FTMS. Protein modification with a double-labeled mix (1:1) of natural abundance isotope (d(0)-BrEA) and 2-bromoethyl-1,1,2,2-d4-amine hydrobromide (d(4)-BrEA), followed by dialysis and trypsin digestion, shows aminoethylated peptides as "twin peptides" separated by four mass units in ESI-FTMS analysis. Using this detection procedure under nondenaturing (native) conditions, C107 is aminoethylated, whereas the four buried thiols remain unlabeled. Aminoethylation of other residues is observed, and correlates with those peptides containing histidine, methionine, and/or the amino terminus. Quantification of the aminoethylation reaction is achieved by labeling with nondeuterated d(0)-BrEA under denaturing conditions following double labeling under native conditions. In addition to complete labeling all five thiols, the intensity of the d(0)-BrEA peak for C107 containing peptides increases, and the change in the d(0)/d(4) ratio between native and denaturing conditions shows 82 +/- 4.5% aminoethylation at C107. This correlation of modification with the recovered activity, indicates that gamma-thia-lysine replaces lysine in the catalytic mechanism. Kinetic constants measured for the rescued K107C mutant enzyme with the substrates fructose 1-phosphate and fructose 1,6-bisphosphate are consistent with the role of the positively charged lysine binding to the C6-phosphate. ESI-FTMS, combined with this double-labeling procedure, allows precise identification of sites and measurement of degree of protein modification.
Figures
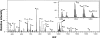

Similar articles
-
Structural insights into the recovery of aldolase activity in N-acetylneuraminic acid lyase by replacement of the catalytically active lysine with γ-thialysine by using a chemical mutagenesis strategy.Chembiochem. 2013 Mar 4;14(4):474-81. doi: 10.1002/cbic.201200714. Epub 2013 Feb 18. Chembiochem. 2013. PMID: 23418011 Free PMC article.
-
Aminoethylation in model peptides reveals conditions for maximizing thiol specificity.Arch Biochem Biophys. 2005 Nov 15;443(1-2):1-10. doi: 10.1016/j.abb.2005.08.020. Epub 2005 Sep 16. Arch Biochem Biophys. 2005. PMID: 16229814
-
A reactive, surface cysteine residue of the class-II fructose-1,6-bisphosphate aldolase of Escherichia coli revealed by electrospray ionisation mass spectrometry.Eur J Biochem. 1995 Jan 15;227(1-2):510-5. doi: 10.1111/j.1432-1033.1995.tb20417.x. Eur J Biochem. 1995. PMID: 7851430
-
Aldolase: a model for enzyme structure-function relationships.Essays Biochem. 1972;8:149-78. Essays Biochem. 1972. PMID: 4564872 Review. No abstract available.
-
Structural characterization of protein-material interfacial interactions using lysine reactivity profiling-mass spectrometry.Nat Protoc. 2023 Aug;18(8):2600-2623. doi: 10.1038/s41596-023-00849-0. Epub 2023 Jul 17. Nat Protoc. 2023. PMID: 37460632 Review.
Cited by
-
Clostridiolysin S, a post-translationally modified biotoxin from Clostridium botulinum.J Biol Chem. 2010 Sep 3;285(36):28220-8. doi: 10.1074/jbc.M110.118554. Epub 2010 Jun 25. J Biol Chem. 2010. PMID: 20581111 Free PMC article.
-
Precise Probing of Residue Roles by Post-Translational β,γ-C,N Aza-Michael Mutagenesis in Enzyme Active Sites.ACS Cent Sci. 2017 Nov 22;3(11):1168-1173. doi: 10.1021/acscentsci.7b00341. Epub 2017 Nov 13. ACS Cent Sci. 2017. PMID: 29202018 Free PMC article.
-
Chemical mutagenesis of vaccinia DNA topoisomerase lysine 167 provides insights to the catalysis of DNA transesterification.Biochemistry. 2013 Feb 5;52(5):984-91. doi: 10.1021/bi301643h. Epub 2013 Jan 23. Biochemistry. 2013. PMID: 23317114 Free PMC article.
-
Structural insights into the recovery of aldolase activity in N-acetylneuraminic acid lyase by replacement of the catalytically active lysine with γ-thialysine by using a chemical mutagenesis strategy.Chembiochem. 2013 Mar 4;14(4):474-81. doi: 10.1002/cbic.201200714. Epub 2013 Feb 18. Chembiochem. 2013. PMID: 23418011 Free PMC article.
-
Modification of residue 42 of the active site loop with a lysine-mimetic side chain rescues isochorismate-pyruvate lyase activity in Pseudomonas aeruginosa PchB.Biochemistry. 2012 Sep 25;51(38):7525-32. doi: 10.1021/bi300472n. Epub 2012 Sep 12. Biochemistry. 2012. PMID: 22970849 Free PMC article.
References
-
- Baranowski, T. and Niederland, T.R. 1949. Aldolase activity of myogen A. J. Biol. Chem. 180 543–551. - PubMed
-
- Beernink, P.T. and Tolan, D.R. 1992. Construction of a high-copy "ATG vector" for expression in Escherichia coli. Protein Exp. Purific. 3 332–336. - PubMed
-
- Benkovic, S.J., Engle, J.L., and Mildvan, A.S. 1972. Magnetic resonance studies of the anomeric distribution and manganese binding properties of fructose phosphates. Biochem. Biophys. Res. Commun. 47 852–858. - PubMed
-
- Bochar, D.A., Tabernero, L., Stauffacher, C.V., and Rodwell, V.W. 1999. Aminoethylcysteine can replace the function of the essential active site lysine of Pseudomonas mevalonii 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochemistry 38 8879–8883. - PubMed
-
- Chen, X., Chen, Y.H., and Anderson, V.E. 1999. Protein cross-links: Universal isolation and characterization by isotopic derivatization and electrospray ionization mass spectrometry. Anal. Biochem. 273 192–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

